Abstract
Formulation and Evaluation of tablet containing combination of Montelukast and Levocetirizine. The objective of present work is-To improve and increase the stability of both montelukast and Levocetirizine in combination as tablet. Enhancement of dissolution of Montelukast. To formulate and evaluate tablets of Levocetirizine and Montelukast for treating allergic rhinitis effectively. The begin with general introduction presenting overview about drugs for allergic rhinitis and combination of montelukast and Levocetirizine tablet. In the present research work, montelukast Levocetirizine tablets were formulated using wet granulation method for the formulation of combined tablet Wet granulation method was employed for the formulation of tablet. Combination tablet with better results is observed in dissolution, assay, drug content. Results of sample formulation was meets with standard specification of IP Stable formulation was prepared. Alkaline excipient was used for increasing the stability of montelukast drug. The formulation of batch F5 was deemed as optimized batch as it shows best results. Formulation of Levocetirizine dihydrochloride and montelukast sodium was prepared for treating allergic rhinitis
Keywords
Levocetirizine and Montelukast, Matrix tablets, wet granulation method
Introduction
1.1 Rhinitis
Allergic rhinitis (AR) causes a collection of symptoms, mostly in the nose and eyes and occurs when one breathes in allergens that leads to its inflammation. Allergic rhinitis is the most common allergic disease worldwide and affects about 18% to 40% of the general population. Persistent allergic rhinitis is an allergic inflammation of the upper respiratory tract due to a year-round encounter with allergens. If left untreated, a chronic state of nasal inflammation accompanied by nasal obstruction can develop and lead to sinusitis, otitis media with effusion, nasal polyps, and asthma Nasal congestion, a major symptom of persistent allergic rhinitis, is difficult to treat Reduction of air passage through the nasal cavities results from the complex network of inflammation and neurogenic phenomena that induce mucosal accumulation of inflammatory cells, engorgement of sinusoidal capacitance vessels. increased permeability of blood vessels, and mucous production. Nasal blockage is troublesome and has considerable impact on quality of life. A vicious cycle starts with nasal congestion which elicits breathing through the mouth, difficulties in falling asleep, night-time awakening, snoring, nasal congestion on awakening with consequent daytime somnolence, impaired mood, poor memory, and decreased productivity at school and work. Patients feel they lack adequate sleep, yet they suffer from insomnia. Sleep impairment leads to an increase in the consumption of sedatives and sleeping medication, which only serves to intensify the problem. The effect of persistent allergic rhinitis on sleep is more pronounced when the condition is moderate-to-severe.(1, 2) Asthma is a chronic lung disease affecting people of all ages. It is caused by inflammation and muscle tightening around the airways, which makes it harder to breathe. Symptoms can include coughing, wheezing, shortness of breath and chest tightness. These symptoms can be mild or severe and can come and go over time. The most common asthma triggers include allergies, air pollution and other airborne irritants, other health conditions including respiratory infections, exercise or physical activity, weather and air temperature, strong emotions, and some medicines. Asthma triggers vary from person to person. An allergy is a reaction by your immune system to something that does not bother most other people. People who have allergies often are sensitive to more than one thing.(3) Substances that often cause reactions are: Pollen. Allergies, also known as allergic diseases, refer to a number of conditions caused by the hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma, and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing, coughing, a runny nose, shortness of breath, or swelling. Note that food intolerances and food poisoning are separate conditions. (4,5)
Common allergens include pollen and certain foods. Metals and other substances may also cause such problems.[11] Food, insect stings, and medications are common causes of severe reactions. Their development is due to both genetic and environmental factors.
MATERIALS AND METHODS
Materials used and their sources:
Matrix tablets of montelukast sodium and levocetirizine dihydrochloride were prepared with Montelukast sodium, levocetirizine dihydrochloride and excipients, Microcrystalline cellulose (MCC), Maize starch, Lactose, Mannitol, PVPK-30, Sodium Lauryl Sulphate, Sodium starch glycolate, Magnesium stearate, Talc, were used for making a tablet. (Collected from Snehal pharma & surgicals pvt. Ltd.)
Pre-formulation study:
- Organoleptic properties of drug:
The organoleptic properties of montelukast and Levocetirizine were studied such as color, odor, appearance, pH and solubility were carried out in various solvents such as alcohol, ether, methanol, ethanol, chloroform, acetone and water.
- Determination of melting point:
The melting point of the drug was determined using capillary tube method.
Procedure:
- One end of the capillary tube was sealed with the help of flame.
- Then sample was filled and placed in the melting point apparatus.
- The melting point of the drug was note and
- Then obtained observed value was compared with the literature value.
- 1.0g of sample was weighted to the nearest 0.001g in a dry porcelain crucible.
- The crucible was ignited in a muffle furnace at 550 °C for 6 hrs, until free from carbon.
- Then cooled in a desicator and weighed.
- The procedure was repeated to a constant weight of a carbon free ash.
- The percentages of the total ash of dry samples were obtained by the following equation:
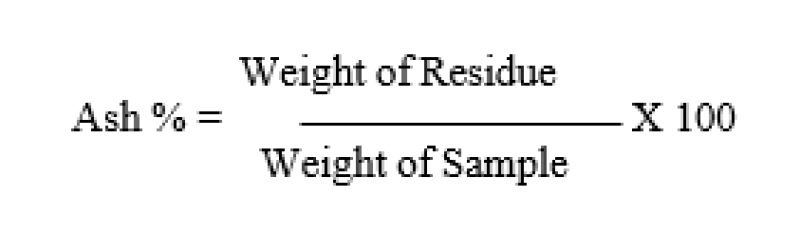
Formulation process:
The first step in the tablet manufacturing process is pre-formulation. During this phase, the active ingredients and excipients (inactive ingredients) that will be used in the tablet are mixed together in the correct proportions. The mixture is then tested to ensure that it has the proper physical and chemical properties.
The next step is the formulation, which is when the actual tablet is created. The mixture from pre- formulation is placed into a tablet press, which forms it into the desired shape and size. Once the tablets are formed, they are usually coated with a film to make them easier to swallow or to prevent them from disintegrating too quickly in the body.
- Granulation
- Compression
- Coating
I. Pre-compression parameters:
a. Angle of Repose:
Angle of repose was determined by using funnel method.
Procedure:
- The blend was poured through funnel that can be raised vertically until a maximum cone height (h) was obtained
- The tip of the funnel should be held close to the growing cone and slowly raised as the pile grows, to minimize the impact of falling particles.
- Then stop pouring the material when the pile reaches a predetermined height.
- And then radius of the heap was measure and angle of repose was calculated using the formula:
? =tan?1 h/r
Where, ? is the angle of repose, h is the height of pile and r is the radius of the base of pile.
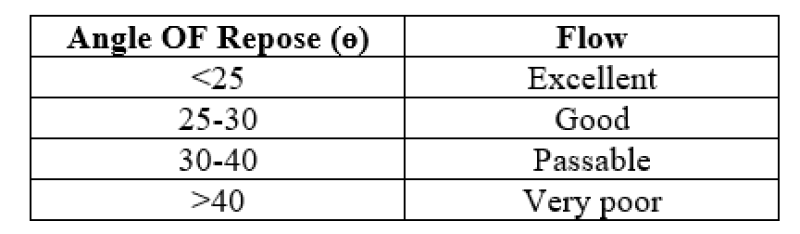
Table No.1: Limits of angle of repose
- Bulk density:
Bulk density of a powder is defined as the ratio of the mass of the powder and its bulk volume. It is used to describe packing of particles.
Procedure:
- For bulk determination, a weighed quantity of the powder material was introduced into a graduated measuring cylinder.
- And then volume of powder was determined.
- Bulk density was calculated using the formula,
Bulk density = Mass of the powder/bulk volume
- Tapped density:
Procedure:
- For determination of the tapped density, a weighed quantity of the powder wasintroduced into a graduated measuring cylinder.
- And then tapped mechanically either manually or using a taping device till a constantvolume was obtained.
- Then the tapped density was calculated using the formula,
Tapped density = Mass of the powder / Tapped volume
- Carr’s compressibility index:
Procedure:
- The simplest way of measurement of free flow of powder is compressibility, anindication of the ease with which a material can be induced to flow.
- The compressibility index is determined by Carr’s index, which is calculated by using the following formula:
- Carr’s Compressibility index = 100 × (1- Bulk density/Tapped density) Where, B is bulk density and T is tapped density
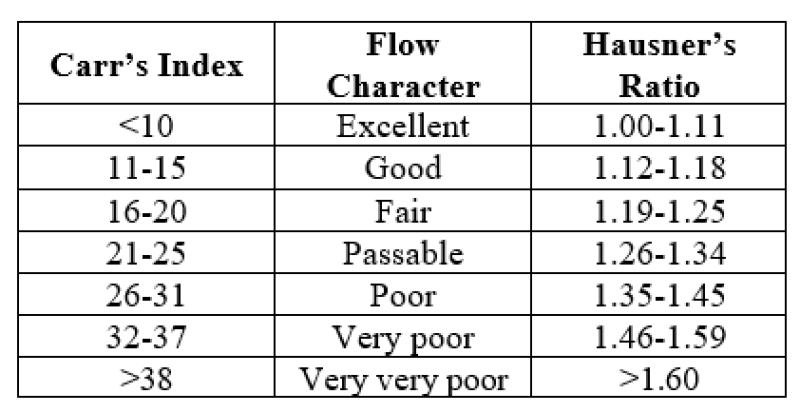
Table No.2: Carr’s compressibility index

- Hausner’s Ratio:
Hausner’s ratio is an index of ease of powder flow. It is calculated by the following formula: Hausner’s Ratio = Tapped density/Bulk density
Lower Hausner’s ratio (< 1>1.25)
Table No.3: Hausner’s ratio and their flow characteristics
- In Process Quality checks during Formulation
Table no.4: IPQC parameter

Equilibrate the column with mobile phase. Inject 20µl of one blank, two replicate injection of standard solution and two replicate injections of sample solution. Check and integrate the peak for Montelukast & Levocetirizine obtained from sample and standard solution.
System Suitability Parameters:
-
- Column efficiency is not less than 2000 theoretical plates.
- Tailing factor is NMT 2.0
- RSD of standard solution is NMT 20
Calculation
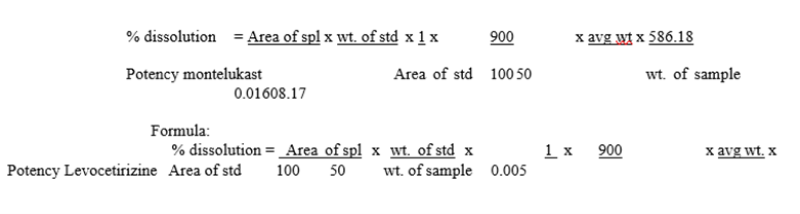
Calculate the content of Montelukast & Levocetirizine by comparing the average area of the chromatograms obtained by the sample solution against that of the standard solution.
DISSOLUTION-
Determined by HPLC (High performance liquid chromatography)
3.6.1 Preparation of Dissolution Media (Quantity 7 liters):
1% w/ Sodium lauryl sulphate: Take 7 liter of water in grated PET bucket. Weigh and transfer 70.0 g of Sodium lauryl sulphate in 2 liters hot water sonicate to dissolve this 2-liter solution transfer in grated bucket and make up the volume up to in 7 liters of water, Stir the solution by Teflon rod for 5 minutes.
Table:5 -Dissolution Condition:
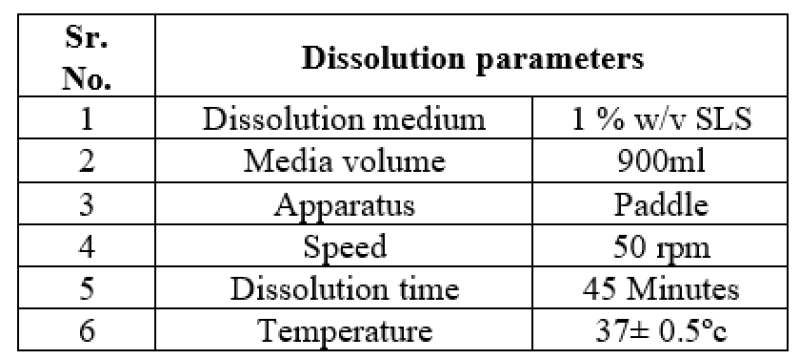
Transfer 900 ml of the dilution media in six dissolution vessels, set the dissolution apparatus for Montelukast and Levocetirizine tablets programmed which are already saved in system, weigh six (individual tablets, drop the tablets into each six dissolution vessels containing dissolution media after system display show really message on screen, Run the dissolution test apparatus as per above mentioned condition with 10 ml of sample from each dissolution vessels using bend cannula. Filter the solutions through a Whatman fibre paper into test tubes.
- Preparation of Standard Stock Solution A (Montelukast)
Weigh and transfer Equivalent to 57mg of Montelukast sodium working standard into 100ml volumetric flask and add 50ml, of Methanol, sonicate to dissolve fie 10 minutes and make up volume up to mark with Methanol.
- Preparation of Standard Stock Solution B Levocetirizine:
Weigh and transfer 28mg of Levocetirizine working standard into 100ml volumetric flask add 50ml of Methanol, sonicate to dissolve for 10 minutes and make up volume up to mark with Methanol.
- Preparation of Standard Solution
Pipette out 1 ml of the standard stock solution A (Montelukast) and standard stock solution B (Levocetirizine) solution to 50ml with the disso media
- Chromatographic condition:
Same as described in Assay, using 100 µl injection volumes.
Calculations
Calculate the content of Montelukast and Levocetirizine by comparing the average area obtained by the sample solution against that of the standard solution.
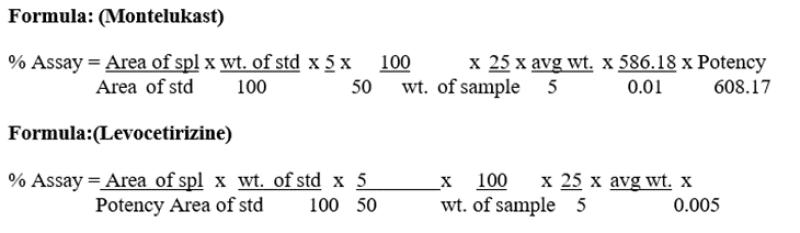
FORMULA
RESULT AND DISCUSSION
- Pre-formulation study:
Identification and compatibility studies of drug and excipients:
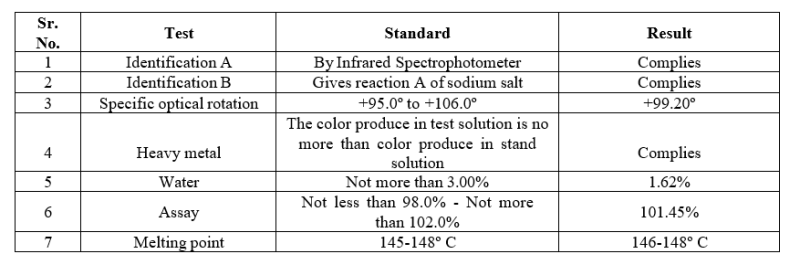
Name of the material: Montelukast sodium USP
Table no. 6: Identification and compatibility of montelukast sodium
Name of the material: Levocetirizine Dihydrochloride USP/IP
Table no. 7: Identification and compatibility of Levocetirizine
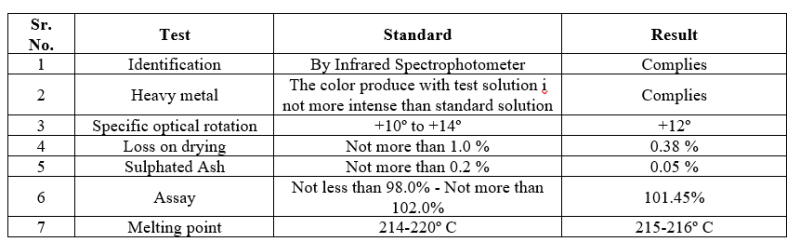
The identification and compatibility studies of drug and excipient were complying with standard specification.
- Organoleptic properties of drug:
Name of the material: Montelukast sodium USP
Table no.8: Organoleptic properties of Montelukast sodium

Name of the material : Levocetirizine Dihydrochloride USP/IP
Table no.9: Organoleptic properties of Montelukast sodium

The Organoleptic properties of drugs were studies and it complies with the standard specification.
- Formulation process
- Granulation
Formulation table of Montelukast and levocetirizine tablets
Composition and formulation of Montelukast and levocetirizine tablets by using Sodium Laurel Sulphate are
Table No.10: Formulation Table of Montelukast and levocetirizine tablets
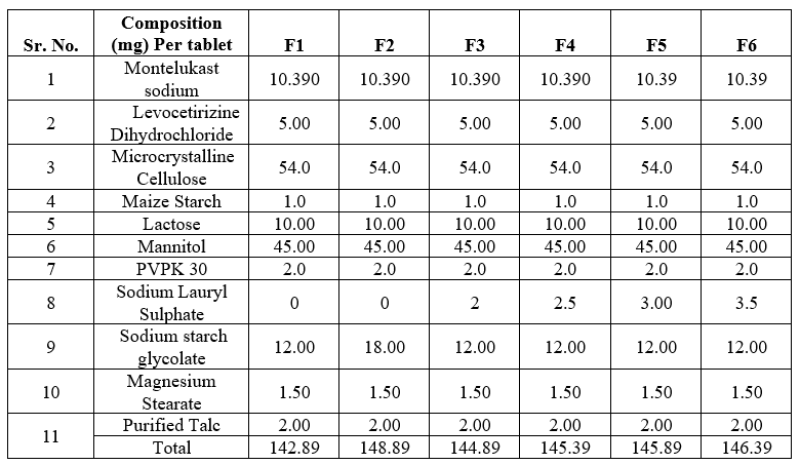
*All the quantities are in mg and for one tablet.
II. Tablet Compression
Given below are the parameters with standard specification mentioned and results of these parameters were complying with standard specification.
Table No.11: Tablet compression parameters
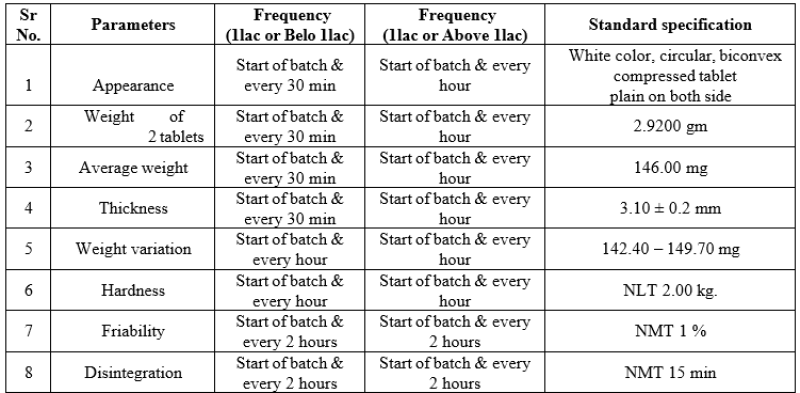
- Coated tablet IPQC
The tablets of each batch were evaluated for various tests namely weight variation, hardness, thickness, % friability, disintegration time, drug content and % drug release. The results were
Table No.12: Coated tablet IPQC parameter
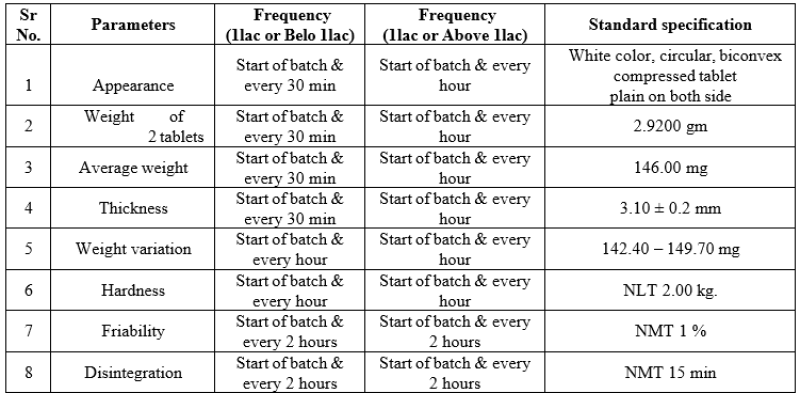
From the above results it was observed that the formulation F5 showed best results and was further used for optimization. The results are given as follows [weight of 20 tablets (2.916 gm), average weight(145.80 mg), hardness (3.50± 5.00 kg/m?2;), thickness (3.05-3.28 mm), % friability (0.22%) disintegration time (8-
IV. Dissolution Study
V. It was determined by HPLC (High performance liquid chromatography)
Dissolution of drug and % release of drug after 45 min.
Table no. 13: % release of drug after 45 min

With the mentioned % release of drug Levocetirizine dihydrochloride and montelukast sodium were under standard specification.
These results of dissolution showing better results with long stability.
VI. Study of optimized batch:
- Granulation
Dried granules reconciliation after granulation
Table no. 14: Dried granules reconciliation after granulation

1. Theoretical weight of granules : 5.600Kg
2. Actual weight of granules : 5.550Kg
Percentage yield :
Calculation : 2/1 * 100
= 99.11%
The % yields of granules after granulation were calculated and it is under the range and result was found to be satisfactory.
• Pre-compression parameters:
Flow properties of the tablet blend tests like bulk density, tapped density, angle of repose, Hausner’s ratio and Carr’s index were performed and the results are given below.
Table no. 15: Pre-compression parameter of blend

Dried granules reconciliation after Lubrication
Table no. 16: Dried granules reconciliation after Lubrication

1. Theoretical weight of granules: 7.165 Kg
2. Actual weight of granules: 7.150Kg
3. Percentage yield:
Calculation: 2/1 * 100
= 99.79%
The % yield of granules after lubrication were calculated and it is under the range and result was found to be satisfactory
METHOD OF ANALYSIS
- 1. AVERAGE WEIGHT
Weigh accurately 20 tablets on weighing balance and divide the total weight of tablet by the numbers of its.
Table no. 17: Average weight of Tablets
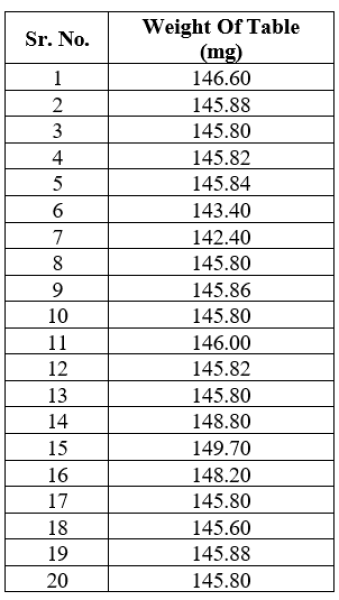
Table no. 18: Physical parameter of tablets

The results are given as follows [average weight(145.80 mg), hardness (3.50± 5.00 kg/m?2;), thickness (3.05-3.28 mm), % friability (0.22%) disintegration time (8-9 min) % friability (0.22%). Weight variation was determined by weighing 20 tablets of each formulation on an electronic balance. The hardness of tablet is defined as the force applied across the diameter of the tablet in order to break the tablet. The resistance of the tablet to chipping, abrasion or breakage under the condition of storage transformation and handling before usage depends on its hardness. Friability was determined by testing 6.5g of tablets in a friability tester. Accurately weighed ten tablets were placed in Friabilator and rotated at 25 rpm for 4 min. Tablet was showing friability testing NMT 1%. The values of thickness and disintegration were satisfactory in range.
IDENTIFICATION
Identification was determined by comparing peak on chromatogram obtained with test solution with peak in chromatogram with reference solution.
DISSOLUTION
It was determined by HPLC (High performance liquid chromatography
% release of drug after 45 min:
Table no. 19: % release of drug

The % dissolution was determined by HPLC which was found to be satisfactory with specification
REFERENCES
- Rathod R, Misra D. FDC of montelukast with levocetirizine: focus on bilayer technology. Journal of the Indian Medical Association. 2009;107(8):562-4.
- Krishnamoorthy M, Mohd Noor N, Mat Lazim N, Abdullah B. Efficacy of montelukast in allergic rhinitis treatment: a systematic review and meta-analysis. Drugs. 2020;80(17):1831-51.
- Small P, Keith PK, Kim H. Allergic rhinitis. Allergy, asthma & clinical immunology. 2018;14(2):1-11..
- Ciebiada M, Górska-Ciebiada M, DuBuske LM, Górski P. Montelukast with desloratadine or levocetirizine for the treatment of persistent allergic rhinitis. Annals of allergy, asthma & immunology. 2006;97(5):664-71.
- Agha ds, el-zien h. Solid state compatibility studies between montelukast sodium and levocetirizine. Asian J Pharm Clin Res. 2018;11(3):368-74.
- Gupta V, Matreja PS. Efficacy of montelukast and levocetirizine as treatment for allergic rhinitis. J Allergy Ther. 2010;1(1)
- Mohammed M, Maringanti PS, Mamidi S. Formulation and evaluation of bilayered tablets of montelukast and levocetrizine dihydrochloride using natural and synthetic polymers. International Journal of Drug Delivery. 2011;3(4):597.
- Woods L, Craig TJ (2006) The importance of rhinitis on sleep, daytime somnolence, productivity and fatigue. Curr Opin Pulm Med 12: 390-396.
- Bousquet J, Lund VJ, van Cauwenberge P, Bremard-Oury C, Mounedji N, et al. (2003) Implementation of guidelines for seasonal allergic rhinitis: a randomized controlled trial. Allergy 58: 733-741.
- Lagos JA, Marshall GD (2007) Montelukast in the management of allergic rhinitis. Ther Clin Risk Manag 3: 327-332.
- Gonyeau MJ, Partisano AM (2003) A clinical review of montelukast in the treatment of seasonal allergic rhinitis. Formulary 38: 368-378.
- Storms W (2008) Allergic rhinitis-induced nasal congestion: its impact on sleep quality. Prim Care Resp J 17: 7-18.
- BousquetJ, van Cauwenberge P, Khaltaev N (2001) Allergic rhinitis and its impact on Asthma. J Allergy Clin Immunol 108: 147-336.
- Camelo-Nunes IC (2006) New antihistamines: a critical review. J Pediatr (Rio J) 82: S173-S180.
- Meltzer EO, Prenner BM, Nayak A, Desloratadine Study Group (2001) Efficacy and tolerability of once-daily 5 mg desloratadine, an H1 receptor antagonist, in patients with seasonal allergic rhinitis. Clin Drug Invest 21: 25-32.
- Brunton LL, Lazo JS, Parker KL (2006) Histamine, Bradykinin and Their antagonist: Goodman and Gilman’s The pharmacological Basis of Therapeutics. (11th edn) New York: McGraw-Hill.
- Braido F, Riccio AM, Rogkakou A, Massacane P, Guerra L, Fum-agalli F, et al. Montelukast efects


 Dipali Kakade* 1
Dipali Kakade* 1
 Shabnam khan 2
Shabnam khan 2
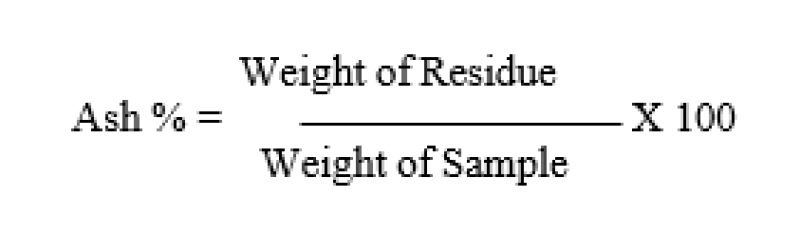


















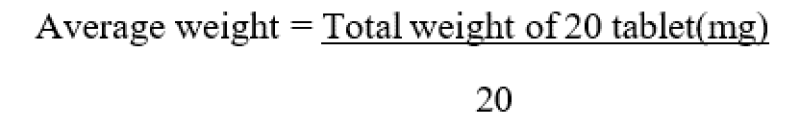


 10.5281/zenodo.10794492
10.5281/zenodo.10794492