Abstract
This comprehensive review explores the latest advancements in the field of alcohol withdrawal, from elucidating the neurobiological basis to innovating therapeutic strategies. The intricate mechanisms of alcohol dependence, shaped by chronic alcohol exposure and neuroadaptive changes, set the stage for withdrawal symptoms. Unraveling these processes at the molecular and cellular levels, including the role of neurotransmission, neuroinflammation, and epigenetic modifications, provides a nuanced understanding of alcohol withdrawal. The integration of emerging technologies, such as advanced neuroimaging and omics approaches, facilitates the identification and validation of novel biomarkers. This not only enhances diagnostic and prognostic capabilities but also opens avenues for personalized medicine. Therapeutic approaches, encompassing both pharmacological and non-pharmacological interventions, showcase the evolving strategies to address the multifaceted challenges of alcohol withdrawal. However, persistent challenges include the limitations of current research, ethical considerations, and potential barriers to implementing innovative therapeutic strategies. Future research directions focus on precision medicine, neurobiological mechanisms, and long-term outcomes. The collaborative effort to navigate these new frontiers holds promise for transforming the landscape of alcohol withdrawal and improving patient outcomes.
Keywords
Alcohol withdrawal, Neurobiological mechanisms, Emerging technologies, Biomarkers, Personalized medicine, Pharmacological interventions, Non-pharmacological interventions, Precision medicine, Therapeutic strategies, Future directions
Introduction
- Background and significance of alcohol withdrawal
Alcohol withdrawal, stemming from the abrupt reduction or cessation of alcohol intake in individuals with alcohol dependence, encompasses a range of physiological and psychological challenges. From mild discomfort to life-threatening conditions like delirium tremens, the prevalence of alcohol use disorder underscores the need for in-depth exploration. The neurobiological basis of alcohol dependence involves complex changes in neurotransmitter systems, neural circuits, and molecular signaling pathways. Chronic alcohol exposure induces adaptations in the central nervous system, leading to a state of dependence. Understanding the mechanisms behind these adaptations is crucial in addressing the subsequent withdrawal symptoms. [1-6]
B. Brief Overview of Existing Treatments and Their Limitations
Current treatments for alcohol withdrawal predominantly involve medications such as benzodiazepines, antipsychotics, and anticonvulsants. While effective in managing acute symptoms, these medications have drawbacks including the risk of dependence, potential for abuse, and side effects. Behavioral interventions play a key role in long-term recovery, but they often complement rather than specifically target withdrawal symptoms. Limitations in current treatments highlight the need for innovative approaches. [7]
C. Importance of Exploring New Frontiers in Understanding and Treating Alcohol Withdrawal
Advancements in neuroscience, technology, and personalized medicine present unprecedented opportunities to unravel the intricacies of alcohol withdrawal. Neuroimaging, omics technologies, and biomarker discovery offer new tools to explore withdrawal at molecular and cellular levels. This understanding could lead to targeted and more effective therapeutic interventions. [8]
Exploring new frontiers is crucial for enhancing treatment precision and minimizing adverse effects associated with existing medications. A comprehensive understanding of alcohol withdrawal's neurobiological underpinnings opens avenues for novel pharmacological and non-pharmacological interventions, improving care for individuals grappling with alcohol dependence. This introduction underscores the importance of advancing our knowledge and treatment approaches in light of the current limitations in the field.
II. Mechanisms of Alcohol Withdrawal
A. Neurobiological Basis of Alcohol Dependence
1. Effects of Chronic Alcohol Exposure on the Brain
Chronic alcohol exposure induces substantial alterations in the central nervous system, particularly within regions associated with reward, motivation, and cognition. These changes encompass structural and functional modifications involving neurotransmitter systems, neural connectivity, and receptor expression. Notably, the mesolimbic dopamine system, a key component of the brain's reward pathway, undergoes adaptations contributing to the development of alcohol dependence. [9]
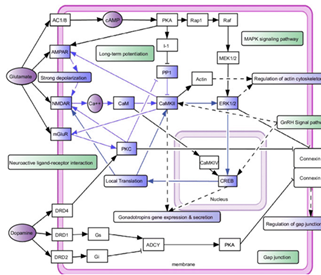
Figure 1: Schematic representation of the neurobiological basis of alcohol dependence
Figure 1 illustrate the CAMKII activation is pivotal in addiction development, fostering fast and slow positive feedback loops that interconnect through signal transduction and transcription processes, involving functional modules such as cytoskeleton regulation and gene expression. These pathways highlight the multifaceted dynamics in addiction states.
2. Neuroadaptive Changes Leading to Withdrawal Symptoms
The neuroadaptive responses triggered by prolonged alcohol exposure establish a state of dependence. Abrupt cessation of alcohol consumption prompts these adaptations to react, giving rise to withdrawal symptoms. These symptoms range from mild manifestations such as anxiety and irritability to more severe outcomes like seizures and delirium tremens. A comprehensive understanding of these neuroadaptive changes is vital for unraveling the mechanisms that underlie alcohol withdrawal. [10]
B. Molecular and Cellular Mechanisms Underlying Withdrawal
1. GABAergic and Glutamatergic Neurotransmission
Alcohol's impact on GABAergic and glutamatergic neurotransmission is pivotal in withdrawal. Chronic alcohol use enhances inhibitory GABAergic transmission while dampening excitatory glutamatergic signaling. The sudden cessation of alcohol results in an imbalance between these systems, contributing to hyperexcitability and the emergence of withdrawal symptoms. Targeting these neurotransmitter pathways holds promise for potential pharmacological interventions.Figure 2 shows the molecular and cellular mechanisms underlying alcohol withdrawal, highlighting the interplay between GABAergic and glutamatergic neurotransmission [11].
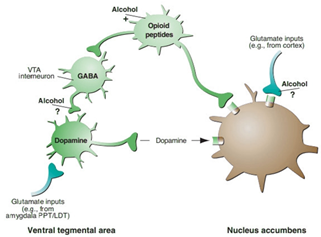
Figure 2: Molecular and cellular mechanisms underlying alcohol withdrawal
2. Role of Neuroinflammation in Withdrawal
Neuroinflammation, characterized by the activation of the immune system in the central nervous system, is increasingly recognized as a significant contributor to alcohol withdrawal. Chronic alcohol exposure triggers inflammatory responses, and withdrawal exacerbates these processes. Microglial activation, cytokine release, and alterations in neuroimmune signaling contribute to neuronal dysfunction, potentially underpinning the severity of withdrawal symptoms. Investigating anti-inflammatory strategies offers novel avenues for therapeutic interventions.[12]
3. Epigenetic Modifications and Their Impact on Withdrawal
Epigenetic modifications, including DNA methylation, histone acetylation, and microRNA regulation, play a pivotal role in the enduring effects of alcohol exposure and withdrawal. These modifications influence patterns of gene expression, contributing to persistent changes in neural circuits. Deciphering the epigenetic landscape of alcohol withdrawal provides insights into the lasting consequences of alcohol dependence and identifies potential targets for interventions aimed at mitigating withdrawal symptoms. [13]
III. Emerging Technologies and Methodologies
A. Advances in Neuroimaging Techniques
1. Functional Magnetic Resonance Imaging (fMRI)
Recent advances in functional magnetic resonance imaging (fMRI) have provided researchers with a powerful tool to investigate the dynamic changes in brain activity associated with alcohol withdrawal. fMRI allows for the real-time visualization of neural activity by measuring changes in blood flow and oxygenation. Applications of fMRI in alcohol withdrawal research enable the identification of specific brain regions implicated in withdrawal symptoms, shedding light on the functional connectivity and alterations within neural circuits. [14]
2. Positron Emission Tomography (PET)
Positron emission tomography (PET) has emerged as another valuable neuroimaging technique for studying alcohol withdrawal. PET enables researchers to examine molecular processes in vivo by tracking the distribution of radiolabeled compounds. This technology provides insights into neurotransmitter systems, receptor binding, and metabolic activity in the brain during withdrawal. By employing PET imaging, researchers can gain a deeper understanding of the neurochemical changes associated with alcohol withdrawal. [15]
B. Omics Approaches in Understanding Withdrawal Mechanisms
1. Genomics
Genomic studies contribute significantly to unraveling the genetic factors influencing susceptibility to alcohol withdrawal and its severity. Genome-wide association studies (GWAS) and next-generation sequencing techniques allow researchers to identify genetic variations associated with withdrawal symptoms. Understanding the genetic underpinnings of alcohol withdrawal enhances the potential for personalized treatment approaches and the development of targeted interventions.
2. Proteomics
Proteomic analyses provide a comprehensive view of the protein expression patterns associated with alcohol withdrawal. By studying changes in protein profiles, researchers can identify key signaling pathways and molecular mechanisms involved in withdrawal. Proteomics also offer potential biomarkers for predicting and monitoring withdrawal symptoms, aiding in the development of more precise diagnostic tools and therapeutic strategies.
3. Metabolomics
Metabolomics, the study of small molecules involved in cellular processes, offers insights into the metabolic changes occurring during alcohol withdrawal. Metabolomic profiling can identify specific metabolites associated with withdrawal symptoms, providing a holistic view of the physiological alterations induced by alcohol cessation. This information contributes to a better understanding of the systemic impact of alcohol withdrawal and may uncover novel targets for therapeutic intervention. [16]
C. Animal Models for Studying Alcohol Withdrawal
1. Transgenic and Knockout Models
Transgenic and knockout animal models play a crucial role in elucidating the molecular and genetic basis of alcohol withdrawal. By manipulating specific genes related to neurotransmitter systems or neuroadaptive changes, researchers can simulate withdrawal conditions and study the resulting effects. These models contribute valuable data for understanding the mechanistic aspects of alcohol withdrawal at the genetic and molecular levels.
2. Behavioral Assays for Withdrawal Assessment
Behavioral assays in animal models provide a means to assess the subjective and objective manifestations of alcohol withdrawal. These assays include observational measures, cognitive assessments, and tests of anxiety-like behaviors. By employing a range of behavioral assays, researchers can quantitatively evaluate the severity of withdrawal symptoms and the effectiveness of potential interventions, bridging the gap between molecular mechanisms and clinical outcomes. [17]
IV. Biomarkers of Alcohol Withdrawal
A. Identification and Validation of Novel Biomarkers
1. Molecular Markers in Blood and Cerebrospinal Fluid
The identification and validation of novel biomarkers in blood and cerebrospinal fluid have become pivotal in understanding the underlying mechanisms and assessing the severity of alcohol withdrawal. Molecular markers, such as specific proteins, nucleic acids, and metabolites, offer insights into the physiological changes associated with withdrawal. Advances in high-throughput technologies enable the systematic screening of these biological fluids, providing a comprehensive profile of molecular alterations during alcohol withdrawal. The validation of such biomarkers holds promise for the development of diagnostic tools and targeted interventions. [18]
2. Imaging-Based Biomarkers
Imaging-based biomarkers, derived from neuroimaging techniques like fMRI and PET, provide a non-invasive means to assess structural and functional changes in the brain during alcohol withdrawal. These biomarkers offer objective measures of neural activity, connectivity, and structural integrity, contributing to a more nuanced understanding of the neurobiological aspects of withdrawal. The validation of imaging-based biomarkers enhances their potential utility in clinical settings, aiding in the diagnosis, prognosis, and treatment monitoring of individuals undergoing alcohol withdrawal. [19]
B. Potential for Personalized Medicine in Alcohol Withdrawal
1. Genetic Markers Predicting Withdrawal Severity
The exploration of genetic markers holds significant promise for personalized medicine in alcohol withdrawal. Genetic studies, including GWAS and candidate gene approaches, aim to identify specific genetic variations associated with the severity and susceptibility to withdrawal symptoms. By unraveling the genetic landscape of alcohol withdrawal, clinicians can potentially predict an individual's risk and tailor treatment strategies accordingly. The validation of genetic markers may pave the way for precision medicine approaches that consider genetic factors in the management of alcohol withdrawal.
2. Individualized Treatment Based on Biomarker Profiles
The integration of biomarker profiles, encompassing molecular, imaging, and genetic data, opens avenues for individualized treatment strategies in alcohol withdrawal. Tailoring interventions based on a person's unique biomarker signature allows for targeted and more effective therapeutic approaches. This personalized medicine paradigm considers the heterogeneity of withdrawal symptoms among individuals, optimizing treatment outcomes and minimizing adverse effects. The validation of biomarker profiles as predictors of treatment response is crucial for advancing the implementation of individualized care in the context of alcohol withdrawal. [20]
V. Therapeutic Approaches
A. Pharmacological Interventions
1. Existing Medications and Their Limitations
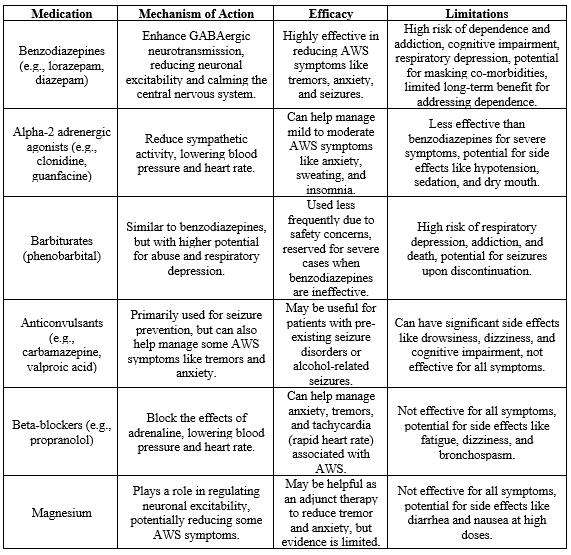
Current pharmacological interventions for alcohol withdrawal primarily involve medications such as benzodiazepines, antipsychotics, and anticonvulsants. While these medications effectively alleviate acute withdrawal symptoms, they come with limitations, including the risk of dependence, potential for abuse, and side effects. Additionally, the one-size-fits-all approach may not address the variability in individual responses to treatment. Recognizing these limitations underscores the need for the exploration of alternative pharmacological interventions that provide targeted and personalized approaches to managing alcohol withdrawal.Table 1 provides existing medications for alcohol withdrawal, detailing their mechanisms of action, efficacy, and limitations [21].
Table 1: Existing medications for alcohol withdrawal
2. Novel Drug Targets and Compounds Under Investigation
Ongoing research is focused on identifying novel drug targets and compounds to enhance the pharmacological management of alcohol withdrawal. Advances in neuropharmacology and our growing understanding of the neurobiological mechanisms involved in withdrawal open doors to new therapeutic possibilities. Investigational compounds may target specific neurotransmitter systems, neuroinflammatory pathways, or other molecular targets implicated in withdrawal. The exploration of these novel drug targets aims to improve the efficacy and safety of pharmacological interventions while minimizing undesirable side effects associated with existing medications. [22]
B. Non-Pharmacological Interventions
1. Cognitive-Behavioral Therapies
Non-pharmacological interventions, particularly cognitive-behavioral therapies (CBT), play a crucial role in addressing the psychological aspects of alcohol withdrawal and promoting long-term recovery. CBT focuses on modifying maladaptive behaviors and thought patterns associated with alcohol use, helping individuals develop coping strategies and resilience. By addressing underlying cognitive processes, CBT contributes to reducing the risk of relapse and enhancing overall psychological well-being during and after the withdrawal phase.
2. Neuromodulation Techniques
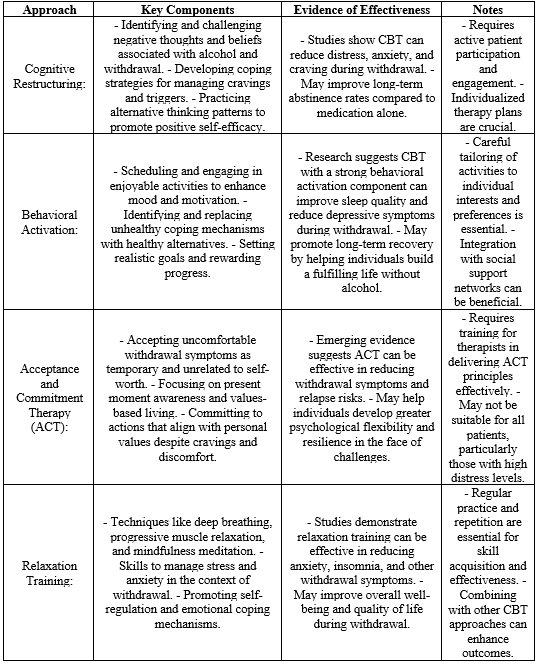
Neuromodulation techniques, such as transcranial magnetic stimulation (TMS) and electroconvulsive therapy (ECT), are emerging as potential non-pharmacological interventions for alcohol withdrawal. These techniques involve the application of electrical or magnetic stimuli to modulate neural activity. Preliminary research suggests their potential in mitigating withdrawal symptoms and influencing neural circuits implicated in addiction. However, further studies are needed to elucidate their safety, efficacy, and optimal application in the context of alcohol withdrawal.Table 2 gives summary of cognitive-behavioral therapies, outlining key approaches, components, and evidence for their effectiveness in alcohol withdrawal [23].
Table 2: Summary of cognitive-behavioral therapies
3. Lifestyle Interventions and Holistic Approaches
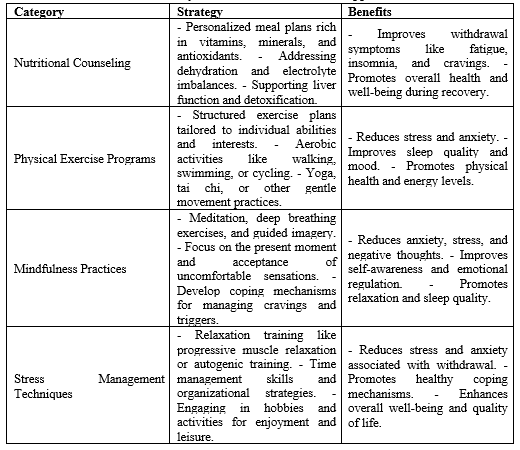
Complementary to traditional treatments, lifestyle interventions and holistic approaches aim to address the broader aspects of an individual's well-being during and after alcohol withdrawal. These may include nutritional counseling, physical exercise programs, mindfulness practices, and stress management strategies. Holistic approaches recognize the interconnectedness of physical, mental, and emotional well-being, aiming to provide a comprehensive and individualized support system for individuals undergoing alcohol withdrawal. Table 3 provides lifestyle interventions and holistic approaches, categorizing strategies such as nutritional counseling, physical exercise programs, mindfulness practices, and stress management in alcohol withdrawal. [24]
Table 3:Lifestyle interventions and holistic approaches
CHALLENGES AND FUTURE DIRECTIONS
A. Limitations of Current Research and Treatment Approaches
Despite significant progress in understanding alcohol withdrawal, there are inherent limitations in current research and treatment approaches. Existing studies often face challenges related to small sample sizes, heterogeneous populations, and variability in individual responses. Moreover, the focus on acute withdrawal symptoms may not fully capture the complexities of long-term recovery. Addressing these limitations is crucial for advancing our understanding of alcohol withdrawal and developing more effective treatment strategies.
B. Ethical Considerations in Experimental Studies
The ethical conduct of experimental studies in alcohol withdrawal research poses important considerations. Issues such as obtaining informed consent, ensuring participant confidentiality, and balancing potential benefits with risks require careful attention. Ethical concerns also extend to vulnerable populations, including individuals with co-occurring mental health disorders or those at risk of severe withdrawal symptoms. Striking a balance between scientific rigor and ethical responsibility is imperative for maintaining the integrity of research in this field.
C. Potential Barriers to Implementing New Therapeutic Strategies
The translation of novel therapeutic strategies from research settings to clinical practice faces potential barriers. Resistance to change within established treatment paradigms, regulatory hurdles, and financial constraints may impede the adoption of innovative interventions. Additionally, challenges in disseminating new approaches to diverse healthcare settings and ensuring accessibility to a broad range of individuals may pose obstacles to the implementation of advancements in alcohol withdrawal treatment. Overcoming these barriers requires collaborative efforts among researchers, clinicians, policymakers, and other stakeholders.
D. Future Research Directions and Unanswered Questions
The landscape of alcohol withdrawal research is dynamic, with several avenues for future exploration. Key areas for further investigation include:
- Precision Medicine:
Advancing our understanding of individual variability in withdrawal responses and tailoring treatments based on biomarker profiles.
- Neurobiological Mechanisms:
Delving deeper into the molecular and cellular mechanisms underlying alcohol withdrawal, including the role of specific neurotransmitter systems, neuroinflammation, and epigenetic modifications.
- Long-Term Outcomes:
Examining the factors influencing sustained recovery beyond the acute withdrawal phase, including the impact of treatment modalities on relapse prevention.
- Technology Integration:
Harnessing the potential of digital health tools, telemedicine, and mobile applications to enhance treatment adherence, monitoring, and support.
- Combination Therapies:
Investigating the efficacy of combining pharmacological and non-pharmacological interventions to address the multifaceted nature of alcohol withdrawal.
As research progresses, unanswered questions regarding the optimal duration of treatment, the influence of comorbid conditions, and the long-term effects of various interventions underscore the need for continued exploration in the field of alcohol withdrawal. [25]
CONCLUSION
The exploration of new frontiers in alcohol withdrawal, spanning from understanding underlying mechanisms to advancing therapeutic strategies, unveils a dynamic landscape marked by significant progress and persistent challenges. The neurobiological basis of alcohol dependence, intricately linked to chronic alcohol exposure and neuroadaptive changes, sets the stage for the emergence of withdrawal symptoms. Investigating these mechanisms at the molecular and cellular levels, including the role of GABAergic and glutamatergic neurotransmission, neuroinflammation, and epigenetic modifications, provides a comprehensive understanding of the complex processes at play during alcohol withdrawal.
The integration of emerging technologies and methodologies, such as advanced neuroimaging techniques, omics approaches, and sophisticated animal models, offers unprecedented opportunities to deepen our knowledge and refine our approach to alcohol withdrawal. These tools allow for the identification and validation of novel biomarkers, shedding light on diagnostic, prognostic, and treatment-related facets of withdrawal. The potential for personalized medicine, driven by genetic markers and individualized treatment based on biomarker profiles, represents a promising direction for tailoring interventions to the unique needs of individuals undergoing alcohol withdrawal. Examining therapeutic approaches reveals the dynamic interplay between pharmacological and non-pharmacological interventions. Existing medications, while effective in managing acute symptoms, present limitations such as the risk of dependence and potential side effects. The exploration of novel drug targets and compounds, coupled with non-pharmacological strategies like cognitive-behavioral therapies, neuromodulation techniques, and lifestyle interventions, underscores the diverse array of tools available for addressing the multifaceted challenges of alcohol withdrawal. However, this journey into new frontiers is not without obstacles. The limitations of current research, ethical considerations in experimental studies, and potential barriers to implementing innovative therapeutic strategies underscore the complexities inherent in advancing the field. Addressing these challenges requires a concerted effort from researchers, clinicians, policymakers, and the broader scientific community. Looking ahead, future research directions beckon, urging exploration into precision medicine, deeper insights into neurobiological mechanisms, and a focus on long-term outcomes. The integration of technology, the investigation of combination therapies, and the pursuit of answers to lingering questions signal a vibrant and evolving field of study. In navigating these new frontiers, the overarching goal remains the enhancement of our understanding and treatment of alcohol withdrawal. By embracing innovation, collaboration, and a commitment to ethical research practices, the scientific community can continue to make strides in transforming the landscape of alcohol withdrawal, ultimately improving the lives of individuals grappling with this complex and challenging condition.
REFERENCES
- Marchand TD, Dunham CM, Chance EA, Hileman BM. Trauma center admission risk conditions and the probability for developing alcohol withdrawal syndrome: A retrospective study. Injury. 2023 Jan 1;54(1):198-206. https://doi.org/10.1016/j.injury.2022.08.072
- Pourmand A, AlRemeithi R, Kartiko S, Bronstein D, Tran QK. Evaluation of phenobarbital based approach in treating patient with alcohol withdrawal syndrome: A systematic review and meta-analysis. The American Journal of Emergency Medicine. 2023 Apr 5.https://doi.org/10.1016/j.ajem.2023.04.002
- Al-Maqbali JS, Al Alawi AM, Al-Mamari Q, Al-Huraizi A, Al-Maqrashi N. Symptoms-triggered approach versus fixed-scheduled approach of benzodiazepines for management of alcohol withdrawal syndrome: Non-randomized controlled trial. Alcohol. 2023 Feb 1;106:10-4.https://doi.org/10.1016/j.alcohol.2022.09.004
- Nishimura Y, Choi H, Colgan B, Kistler H, Mercado F. Current evidence and clinical utility of phenobarbital for alcohol withdrawal syndrome. European Journal of Internal Medicine. 2023 Mar 21.https://doi.org/10.1016/j.ejim.2023.03.006
- Fluyau D, Kailasam VK, Pierre CG. Beyond benzodiazepines: a meta-analysis and narrative synthesis of the efficacy and safety of alternative options for alcohol withdrawal syndrome management. European Journal of Clinical Pharmacology. 2023 Sep;79(9):1147-57. https://doi.org/10.1007/s00228-023-03523-2
- Kranzler HR. Overview of alcohol use disorder. American Journal of Psychiatry. 2023 Aug 1;180(8):565-72.https://doi.org/10.1176/appi.ajp.20230488
- Alberto GE, Klorig DC, Goldstein AT, Godwin DW. Alcohol withdrawal produces changes in excitability, population discharge probability, and seizure threshold. Alcoholism: Clinical and Experimental Research. 2023 Feb;47(2):211-8.https://doi.org/10.1111/acer.15004
- Alshehri FS. A Review of the Characteristics of Clinical Trials and Potential Medications for Alcohol Dependence: Data Analysis from ClinicalTrials. gov.Medicina. 2023 Jun 7;59(6):1101. https://doi.org/10.3390/medicina59061101
- Wei H, Yu C, Zhang C, Ren Y, Guo L, Wang T, Chen F, Li Y, Zhang X, Wang H, Liu J. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomedicine & Pharmacotherapy. 2023 Apr 1;160:114308.https://doi.org/10.1016/j.biopha.2023.114308
- Vatsalya V, Verster J, Sagaram M, Royer AJ, Hu H, Parthasarathy R, Schwandt ML, Kong M, Ramchandani VA, Feng W, Agrawal R. Novel paradigms for the gut-brain axis during alcohol withdrawal, withdrawal-associated depression, and craving in patients with alcohol use disorder. Frontiers in psychiatry. 2023 Sep 29;14:1203362. https://doi.org/10.3389/fpsyt.2023.1203362
- Dharavath RN, Pina-Leblanc C, Tang VM, Sloan ME, Nikolova YS, Pangarov P, Ruocco AC, Shield K, Voineskos D, Blumberger DM, Boileau I. GABAergic signaling in alcohol use disorder and withdrawal: pathological involvement and therapeutic potential. Frontiers in Neural Circuits. 2023;17.https://doi.org/10.3389/fncir.2023.1218737
- Pérez-Reytor D, Karahanian E. Alcohol use disorder, neuroinflammation, and intake of dietary fibers: a new approach for treatment. The American journal of drug and alcohol abuse. 2023 May 4;49(3):283-9. https://doi.org/10.1080/00952990.2022.2114005
- Ahmed R, Blum K, Thanos PK. Epigenetic Effects of psychoactive drugs. Current Pharmaceutical Design. 2023 Aug 1;29(27):2124-39. https://doi.org/10.2174/1381612829666230706143026
- Van Oort J, Diazgranados N, George DT, Horneffer Y, Schwandt M, Goldman D, Momenan R. Preliminary evidence for changes in frontoparietal network connectivity in the early abstinence period in alcohol use disorder: a longitudinal resting-state functional magnetic resonance imaging study. Frontiers in Psychiatry. 2023;14. 10.3389/fpsyt.2023.1185770
- Tollefson S, Stoughton C, Himes ML, McKinney KE, Mason S, Ciccocioppo R, Narendran R. Imaging Nociceptin Opioid Peptide Receptors in Alcohol Use Disorder With [11C] NOP-1A and Positron Emission Tomography: Findings From a Second Cohort. Biological Psychiatry. 2023 Jan 5.https://doi.org/10.1016/j.biopsych.2022.12.022
- Zhang P, Lan X, Fan B, Chen Y, Wei X, Li X, Fan N, Tang C, Lu L. A protocol for the integration of multi-omics bioinformatics: Mechanism of acupuncture as an adjunctive therapy for alcohol use disorder. Frontiers in Neurology. 2023 Jan 5;13:977487. https://doi.org/10.3389/fneur.2022.977487
- Foster TC. Animal models for studies of alcohol effects on the trajectory of age-related cognitive decline. Alcohol. 2023 Mar 1;107:4-11.https://doi.org/10.1016/j.alcohol.2022.04.005
- He K, Wang Y, Xie X, Shao D. Prediction of Proteins in Cerebrospinal Fluid and Application to Glioma Biomarker Identification. Molecules. 2023 Apr 21;28(8):3617. https://doi.org/10.3390/molecules28083617
- Kong L, He Q, Li Q, Schreiber R, Kaitin KI, Shao L. Rapid progress in neuroimaging technologies fuels central nervous system translational medicine. Drug Discovery Today. 2023 Jan 6:103485. https://doi.org/10.1016/j.drudis.2023.103485
- Boness CL, Witkiewitz K. Precision medicine in alcohol use disorder: Mapping etiologic and maintenance mechanisms to mechanisms of behavior change to improve patient outcomes. Experimental and Clinical Psychopharmacology. 2023 Aug;31(4):769. https://doi.org/10.1037/pha0000613
- Alexiou A, King T. Alcohol withdrawal. bmj. 2023 May 31;381. https://doi.org/10.1136/bmj.p951
- Schwasinger-Schmidt T, Preskorn SH. Discovery of New Transmitter Systems and Hence New Drug Targets. InDrug Development in Psychiatry 2023 Mar 17 (pp. 181-193). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-031-21054-9_7
- Qu L, Ma XP, Simayi A, Wang XL, Xu GP. Comparative efficacy of various pharmacologic treatments for alcohol withdrawal syndrome: a systematic review and network meta-analysis. International Clinical Psychopharmacology. 2023 Dec 27:10-97. https://doi.org/10.1097/YIC.0000000000000526
- Pongsavee K, Payakkakom A, Phukao D, Guadamuz TE. Natural recovery from alcohol: a systematic review of the literature 2006–2019. Journal of Substance Use. 2023 Mar 4;28(2):166-71. https://doi.org/10.1080/14659891.2021.2020348
- Fazel M, Soneson E. Current evidence and opportunities in child and adolescent public mental health: a research review. Journal of Child Psychology and Psychiatry. 2023 Dec;64(12):1699-719. https://doi.org/10.1111/jcpp.13889


 Sunita ogale*
Sunita ogale*





 10.5281/zenodo.11386289
10.5281/zenodo.11386289