Abstract
This study aims to develop and evaluate oral buccal patches containing Ambroxol Hydrochloride (AH) for its mucolytic effects. Buccal patches offer advantages such as bypassing first-pass metabolism, sustained drug release, and improved patient compliance. Buccal patches were prepared using the solvent casting method with various polymers including Hydroxypropyl methylcellulose (HPMC), methyl alcohol, citric acid, etc were incorporated as plasticizers to enhance flexibility. The formulated buccal patches demonstrated uniform thickness and weight, satisfactory folding endurance, and appropriate surface pH, indicating good compatibility with the buccal environment. The swelling index and moisture content were within acceptable ranges, ensuring patch integrity and stability. In vitro drug release studies showed a sustained release profile of Ambroxol Hydrochloride over an extended period, suggesting effective prolonged drug delivery. The bioadhesive strength was found to be adequate, ensuring prolonged adhesion to the buccal mucosa for effective drug absorption.
Keywords
Mucolytics, Buccal patch, Oral route, Ambroxol HCl
Introduction
Congestion, characterized by a sensation of blockage, fullness, or limited airflow, is a key symptom associated with various upper respiratory tract conditions such as allergic rhinitis, acute and chronic rhinosinusitis, and nasal polyposis. This condition adversely affects patients' quality of life by disrupting both their sleep and daytime activities. In cases of allergic rhinitis, congestion is particularly troublesome and often the symptom patients most want to avoid. Additionally, congestion can lead to secondary issues involving the paranasal sinuses, ears, throat, voice, and chest, potentially causing throat irritation, headaches, hearing loss, diminished sense of smell, aggravated asthma, problematic snoring, and disrupted sleep. The main cause of nasal congestion in common upper airway disorders in adults is inflammation, which typically results in venous engorgement, increased nasal secretions, and swelling or edema of the tissues. This inflammation impairs airflow and creates a sensation of nasal blockage. As a result, the development of pharmacological treatments for congestion has focused on addressing these underlying mechanisms, such as reducing inflammation with intranasal corticosteroids and alleviating venous engorgement with decongestants. In chronic rhinosinusitis, congestion may also be exacerbated by nasal polyps that protrude into the nasal passage, causing a physical obstruction. Due to the widespread nature of allergic rhinitis and rhinosinusitis, congestion has become a prevalent issue, even when less common causes are ruled out. Many upper airway conditions where congestion is a common symptom—including allergic rhinitis, nonallergic or vasomotor rhinitis, rhinosinusitis, nasal polyposis, and the common cold—are often inadequately treated due to the limited effectiveness of some therapies and safety concerns with others. Therefore, there is a significant unmet need for better treatment options for congestion. This review examines the treatment strategies for congestion related to these common upper airway conditions and also provides a brief look at treatments for less common nasal disorders and surgical interventions for congestion caused by mechanical issues and treatment-resistant chronic rhinosinusitis. (1- 10)
ORAL ROUTE BENEFITS
The oral route of administration is often preferred by patients for several reasons, making it a popular choice for delivering medications like sitagliptin for type 2 diabetes. Here are the key reasons:
Convenience and ease of use
Non-Invasive:
Oral medications do not require injections, which can be painful and intimidating for many patients.
Simple Administration:
Swallowing a pill is straightforward and can be done without medical assistance, making it more convenient for self-administration.
Comfort and compliance
Less Discomfort:
Oral medications avoid the discomfort associated with needles and injections, which can lead to better patient compliance.
Ease of Routine Integration:
Taking a pill can easily be incorporated into daily routines, such as during meals, which enhances adherence to the medication regimen.
Safety and accessibility
Lower Risk of Infections:
The oral route minimizes the risk of infections that can occur with injections if proper sterile techniques are not followed.
Widely Available:
Oral medications can be stored and transported easily, making them more accessible for a broader population, including those in remote areas.
Psychological benefits
Reduced Anxiety:
Many patients experience anxiety or fear related to needles and injections. Oral medications help alleviate this anxiety.
Greater Sense of Normalcy:
Taking oral medication feels more like a routine part of daily life and less like a medical procedure, contributing to a sense of normalcy and control over one’s health.
Pharmacokinetic advantages
Controlled Release Options:
Many oral medications can be formulated as extended-release or controlled-release forms, providing more stable blood levels of the drug and reducing the frequency of dosing.
Gastrointestinal Absorption:
The gastrointestinal tract is designed to absorb nutrients and medications effectively, providing a suitable pathway for drug delivery.
Economic factors
Cost-Effective:
Oral medications are often less expensive than injectable forms, both in terms of production and distribution.
Lower Healthcare Costs:
Reducing the need for healthcare professional involvement in drug administration can lower overall healthcare costs. Overall, the oral route of administration aligns well with patient preferences for comfort, convenience, and ease of use, thereby enhancing medication adherence and overall treatment outcomes.
THE STRUCTURE OF THE ORAL MUCOSA
Structure
The oral mucosa consists of several layers: the outermost is a stratified squamous epithelium (see Figure 1). Beneath this is the basement membrane, followed by the lamina propria and then the submucosa as the innermost layer. The epithelium features a basal cell layer that is mitotically active and progresses through several differentiating intermediate layers before reaching the surface, where cells are eventually shed. The buccal mucosa has an epithelial layer approximately 40-50 cells thick, whereas the sublingual epithelium is slightly thinner. As epithelial cells move from the basal to the superficial layers, they increase in size and become more flattened.

Figure no 1: Structure of oral mucosa
There is a need to create a dosage form that bypasses first-pass metabolism and gastrointestinal degradation. The oral cavity offers a route for administering therapeutic agents for both local and systemic effects, thus avoiding these metabolic and degradative processes. Patches are commonly prepared using the solvent casting method. The oral cavity is easily accessible for self-administration, allows for dose cessation if needed, is safe, and is generally well-received by patients. To prevent issues like swallowing the dosage form or dose dumping, bioadhesive polymers are increasingly used for buccal controlled-release systems. These polymers help to anchor drug-containing particles to the mucosal surface, leading to extended residence time at the site of absorption or action, targeted drug delivery, and enhanced drug concentration gradients due to the direct contact with the mucosal surface. [11-15]

Figure no 2: Schematic representation of oral mucosa
IMPORTANCE OF BUCCAL PATCH
The oral route is widely regarded as the most convenient and preferred method for administering medications. Tablets and capsules are the most common forms of oral solid dosage. However, certain patient groups—such as those who are bedridden, unconscious, paralyzed, pediatric, geriatric, or traveling without access to water—often face challenges in swallowing these traditional dosage forms. Issues like tremors, dysphagia, and difficulty accessing medications as prescribed can occur. The buccal route offers a viable alternative for these patients. This method allows for direct absorption into the systemic circulation via the jugular vein, effectively bypassing first-pass hepatic metabolism and enhancing bioavailability. The process of bioadhesion involves attaching a synthetic natural macromolecule to biological tissue for an extended period. When this interaction primarily occurs with the mucus layer, it is known as mucoadhesion. Buccal patches, which utilize mucoadhesive polymers, can provide a rapid onset of action due to this mechanism.

Figure no 3: Buccal Patch
The buccal patch addresses concerns related to choking and swallowing difficulties. Its ease of use enhances patient compliance. A buccal patch is a non-dissolving, thin matrix dosage form that includes one or more polymer layers containing the drug and other excipients. A key feature of the patch is its mucoadhesive polymer layer, which adheres to the oral mucosa, gums, or teeth, enabling drug release into the oral mucosa, the oral cavity (unidirectional release), or both (bidirectional release). The patch can be removed and discarded after the designated period. The development of buccal patches is significant because they bypass the limitations of traditional administration routes, offer a rapid onset of action by delivering the drug directly into systemic circulation through the oral mucosa via mucoadhesion, and eliminate the first-pass effect. Additionally, their small size and thin profile contribute to improved patient compliance, and they are painless during application. [16-26]
PHARMACEUTICAL APPLICATION
I. Pain Management
Opioid Analgesics:
Buccal patches are used for delivering opioid analgesics (e.g., fentanyl) for rapid pain relief, particularly in chronic pain and cancer patients.
Non-Opioid Analgesics:
Other pain medications can also be delivered for local pain relief within the oral cavity.
II. Hormone Replacement Therapy
Sex Hormones:
Hormones such as testosterone or estradiol can be administered via buccal patches for conditions requiring hormone replacement therapy, providing a steady release and improved bioavailability.
III. Cardiovascular Treatments
Nitroglycerin:
Used for angina pectoris, buccal patches can provide rapid relief by delivering nitroglycerin directly into the bloodstream.
IV. Smoking Cessation
Nicotine Replacement:
Buccal patches can be an effective tool for delivering nicotine in a controlled manner to help individuals quit smoking.
V. Systemic Delivery of Peptides and Proteins
Insulin and Other Peptides:
Buccal patches can be used to deliver peptides and proteins that are otherwise degraded in the gastrointestinal tract, improving their bioavailability.
VI. Treatment of Oral Conditions
Antifungal and Antibacterial Agents:
For treating oral infections like oral thrush, buccal patches can provide localized delivery of antifungal or antibacterial agents.
Anti-inflammatory Drugs:
These can be used to treat inflammatory conditions within the oral cavity.
VII. Neurological Disorders
Antiepileptic Drugs:
Buccal patches can be used to deliver drugs for managing epilepsy, providing a more consistent and controlled release compared to oral tablets.
Parkinson’s Disease: Certain medications for Parkinson’s disease can be administered buccally to manage symptoms more effectively.
LITERATURE REVIEW
Shewale Vaibhav L,et al. (2021)
The aim of this article is to study the buccal patches. Buccal patch is a nondissolving thin matrix modified release dosage form composed of one or more polymer films or layers containing the drug and/or other excipients. Buccal patches have been become an interesting area of novel drug delivery system as the dosage forms designed for buccal administration should not cause irritation and should be small and flexible enough to be accepted by the patient. The study of buccal patches include its introduction, types of buccal patches, advantages, limitation, potential uses of buccal patches, polymer used, methods of preparation, evaluation.
Aswathy Bose et al.(2020)
The aim of present work was to formulate and evaluate Sitagliptin buccal patch using solvent casting method. Buccal patch gained importance as it overcomes the limitations of current routes of administration, provides rapid onset of action by releasing drug directly to systemic circulation through oral mucosa by mucoadhesion. The formulated buccal patches were evaluated for various parameters like film thickness, surface pH, folding endurance, weight variation, % moisture loss, tensile strength, % elongation, drug content uniformity, and in vitro dissolution studies The optimized formulation (F4) containing HPMC E5 and Eudragit RL 100 polymer combination in 1:1 ratio showed highest in vitro dissolution (99.7 %) and satisfactory stability
Alan T. Cruchely, et.al (2018)
The structure and function of the human oral mucosa have been extensively studied at the level of the constituent tissues and cells over many decades and more recently the molecular biology of this complex biological network has become the subject of more modern multifactorial analyses. The unique aspects of the tissues, its embryological relationship with other mucosa tissues and its position as a gateway for a huge influx of microbial and other antigenic materials have revealed the duality of the gatekeeper/housekeeper function of the oral mucosa and mark it out as far more than the proximal end of the alimentary canal. The purpose of this chapter is to briefly review the structural features of the oral mucosa and place them in the context of the barrier and protective functions that maintain oral health and to act as a reference for the subsequent chapters on the oral mucosa in health and disease.
Pradeep Kumar Koyi, et al. (2013)
Buccal route is an attractive route of administration for systemic drug delivery and it leads direct access to the systemic circulation through the internal jugular vein bypasses drugs from the hepatic first pass metabolism provides high bioavailability. Buccal bioadhesive films, releasing topical drugs in the oral cavity at a slow and predetermined rate, provide distinct advantages over traditional dosage forms for treatment of many diseases. This article aims to review the recent developments in the buccal adhesive drug delivery systems to provide basic principles to the young scientists, which will be useful to circumvent the difficulties associated with the formulation design.
Eli O Meltzer,et al. (2010)
Congestion, as a symptom of upper respiratory tract diseases including seasonal and perennial allergic rhinitis, acute and chronic rhinosinusitis, and nasal polyposis, is principally caused by mucosal inflammation. Though effective pharmacotherapy options exist, no agent is universally efficacious; therapeutic decisions must account for individual patient preferences. Oral H1-antihistamines, though effective for the common symptoms of allergic rhinitis, have modest decongestant action, as do leukotriene receptor antagonists. Intranasal antihistamines appear to improve congestion better than oral forms. Topical decongestants reduce congestion associated with allergic rhinitis, but local adverse effects make them unsuitable for long-term use. Oral decongestants show some efficacy against congestion in allergic rhinitis and the common cold, and can be combined with oral antihistamines. Intranasal corticosteroids have broad anti-inflammatory activities, are the most potent long-term pharmacologic treatment of congestion associated with allergic rhinitis, and show some congestion relief in rhinosinusitis and nasal polyposis. Immunotherapy and surgery may be used in some cases refractory to pharmacotherapy. Steps in congestion management include
- Diagnosis Of The Cause(S)
- Patient Education And Monitoring
- Avoidance Of Environmental Triggers Where Possible
- Pharmacotherapy
- Immunotherapy (For Patients With Allergic Rhinitis) Or Surgery For Patients Whose Condition Is Otherwise Uncontrolled
AIM
The aim of this study is to prepare and evaluate oral buccal patches of Ambroxol Hydrochloride for their potential use as a mucolytic. This involves the formulation of the patches, characterization of their physicochemical properties, and evaluation of their in vitro drug release, mucoadhesive strength, and stability.
OBJECTIVES
- Formulation of Buccal Patches
Develop buccal patches containing Ambroxol Hydrochloride using suitable polymers.
- Characterization
Assess the physicochemical properties such as thickness, weight uniformity, surface pH, folding endurance, and drug content.
- Mucoadhesive Strength
Evaluate the mucoadhesive strength of the patches to ensure they adhere adequately to the buccal mucosa.
- In Vitro Drug Release
Study the in vitro release profile of Ambroxol Hydrochloride from the buccal patches.
- Stability Studies
Conduct stability studies to ensure the patches maintain their integrity and efficacy over time
PLAN OF WORK

Figure no 4: Plan of work
DRUG AND EXCIPIENTS PROFILE
DRUG PROFILE
Ambroxol Hydrochloride

Figure no 5: Ambroxol HCl
IUPAC Name:
hydrogen 4-{[(2-amino-3,5-dibromophenyl) methyl] amino} cyclohexan-1-ol chloride
Boiling point:
468.6°C
Melting point:
235-242°C
Formula:
C13H19Br2ClN2O
Molar mass:
378.1028 g/mol
Routes of administration:
Oral Route, inhaled, intramuscular, intravenous
Type:
Anti- cough agents
Class:
Mucolytics
Half life :
08 to 12 hrs
Shelf life:
5 years
MECHANISM OF ACTION
It breaks down the acid mucopolysaccharide fibres which makes the sputum thinner and less viscous and therefore promotes mucus clearance. It also stimulates synthesis and release of surfactant by type II pneumocytes.

Figure no 6: Mechanism of action of Ambroxol HCl
EXCIPIENTS:
HPMC:
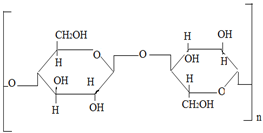
Figure no7: HPMC
IUPAC Name:
Cellulose, 2-hydroxypropyl methyl ether
Formula:
C56H108O30
Soluble in:
Water
Boiling point:
1,102 °C
Molar mass:
1261.4 g/mol
Uses:
- Bioadhesive Agent
- Controlled Release
- Film Forming Agent
Eudragit RL 100:

Figure no 8: Eudragit RL 100
IUPAC Name:
Poly (methacrylic acid-co-ethyl acrylate) 1:1.
Formula:
C11H21NO4
Molecular mass:
32000 g/mol
Soluble:
Methanol
IUPAC Name:
Poly (methacrylic acid-co-ethyl acrylate) 1:1.
Uses:
- Controlled Drug Release:
- Bioadhesion
- Permeability
Methyl Alcohol:

Figure no 9: Methyl alcohol
IUPAC Name:
Methanol
Formula:
CH?OH
Molar mass:
32.04 g/mol
Boiling point:
64.7 °C
Uses:
- Solvent for Polymer Preparation
- Extraction and Purification
- Cleaning and Sterilization
Citric Acid:

Figure no 10: Citric Acid
Formula:
C?H?O?
Molar mass:
192.124 g/mol
Soluble in:
Water, Acetone, Dimethyl sulfoxide, Ethyl acetate
Uses:
- pH Adjustment
- Buffering Agent
- Permeation Enhancer
Glycerol:

Figure no 11: Glycerol
IUPAC ID:
propane-1,2,3-triol
Formula:
C?H?O?
Molar mass:
92.09382 g/mol
Density:
1.26 g/cm?3;
Boiling point:
290 °C
Uses:
- Plasticizer
- Humectant
- Solvent
List of Material
Table no 1: List of materials

List of Instrument
Table no 2: List of Instrument

Experimental Work:
Preformulation studies
A preformulation study is an essential step in drug development where the physical and chemical properties of a drug substance are evaluated before formulating it into a dosage form. This helps in understanding its behavior, stability, and compatibility with excipients, aiding in the design of an effective and stable formulation.
Melting Point:
Take a capillary tube and close its one end by heating the end in the flame for 2-3 minutes while continuously rotating it. Take Ambroxol Hydrochloride fine powder. Dip the open end of the capillary tube in the finely powdered Ambroxol Hydrochloride. Gently tap the capillary tube on the table to fill the compound in the capillary tube to about a length of 1–2 cm. Attach the capillary to the thermometer with the rubber band and dip the thermometer in Thieles tube containing paraffin oil. Keep continuous watch of the temperature and note the temperature as soon as the substance starts to melt.
Fourier transform infrared spectroscopy (FT-IR):
FTIR spectrum was used as an analytical technique for identification of pure drug sample. The spectra for the sample were recorded using a Bruker Vertex 70 FTIR spectrophotometer by KBr pellet method. The samples were analysed by mixing with potassium bromide (1:10) individually and pressed to form a thin pellet by applying pressure using KBr press. The formed pellets were placed within the sample holder. Spectral scanning was taken in the wavelength region between 4000-400 cm?1. FTIR scans of Sitagliptin phosphate were recorded
Spectroscopical analysis:
Determination of Lambda max by UV Spectroscopy:
For determining Lambda max of Ambroxol Hydrochloride ,10 mg of drug was dissolved in Ethanol and diluted to 100 ml to form strength of 100 ?g/ml with the same solvent. It was then scanned in the range of 400 to 200 nm using Ethanol as a blank using UV-Visible spectrophotometer (Shimadzu UV-1900i UV-V is Spectrophotometer) and the maximum wavelength will be determined.
Preparation of calibration curve:
Calibration curve of Ambroxol Hydrochloride was prepared with the help of UV spectroscopy.
Calibration curve of Ambroxol Hydrochloride was prepared in Ethanol.
Calibration curve:
- Preparation of stock solution:
Accurately weighed 10 mg of Ambroxol Hydrochloride was transferred in 100 ml volumetric flask. The drug was dissolved and diluted upto the mark with water to give a solution with concentration of 100 ?g/ml.
- Preparation of working solution:
Appropriate aliquots from stock solution of Ambroxol Hydrochloride (0.2, 0.4, 0.6, 0.8, 1 and 1.2 ml) were accurately withdrawn in 10 ml volumetric flask and diluted upto the mark with Ethanol to obtain the final concentration of solution in range of 2-12 ?g/ml and scanned at Lambda max. Absorbance of these solutions of Ambroxol Hydrochloride were recorded at their Lambda max using Ethanol as blank.
Method of preparation
Solvent casting method
- Buccal patch was prepared by solvent casting method. Polymers like HPMC, and Eudragit RL 100 were used to formulate buccal patch using Glycerol as plasticizer.
- Citric acid was used as saliva stimulating agent, Saccharin sodium as sweetening agent. Formulations F1 to F5 were prepared using different concentrations and ratios of HPMC and Eudragit RL 100.
- The formulation design of Ambroxol HCl buccal patch is given in Table.
- The specified amount of polymers HPMC and Eudragit RL100 was dissolved in solvent methanol.
- The required amount of plasticizer was added to patch forming solution.
- Drug was dissolved in small amount of solvent and added to polymer solution
- Sweetener and saliva stimulating agent were added one by one into above solution with continuous stirring to form a clear aqueous solution.
- The solution was kept undisturbed until the air bubbles get removed. The aqueous solution was poured into the petridish and was air dried.
- The patch was carefully removed from the Petridish and cut into required size suitable for testing.
- The formulated patch was further stored in desiccators for 2 days and wrapped in aluminum foil and packed in self-sealing covers.
Table no 3: Batch formulation

Evaluation Studies
FT-IR Study
The IR spectra were recorded using FTIR spectrophotometer. The samples were prepared by mixing the drug and the excipients in 1:1 ratio and the mixtures were stored in closed containers for 1 week. FTIR spectrum of the samples was taken using pottasium bromide Pellet technique. The physical mixtures of Ambroxol HCl and excipients were scanned in the wavelength region between 3800 and 650 cm-1 and compared to check compatibility of drug with excipient.
Folding endurance
Folding endurance was determined by repeatedly folding the patch at the same place till it breaks. The value of folding endurance obtained from the number of times it folded without breaking.[27]
Weight Variation
Individually weighing 10 randomly selected patches and then calculating average weight to determine weight variation. Digital weighing balance was used to measure each patch. The S.D of weight11 was computed from the mean value. [28-29]
Surface of Ph
The pH meter was calibrated using buffer of pH 4.0 and 7.0 before taking measurement. The patches to be tested were moistened using phosphate buffer pH 6.8 in a petridish and kept for 30 sec. The pH of the formulation was noted after bringing the electrode of pH meter in contact with the surface and allowed to equilibrate for 1 min.
Drug content Uniformity
To determine drug content uniformity, 10 dosage units were individually assayed. The patch was then transferred into a graduated flask, dissolved in 100 ml methanol and the flask was shaken continuously. The solution was filtered after suitable dilutions with methanol and the absorbance was measured at 267 nm using UV spectrophotometer and the drug content was calculated. [30-31]
Percentage Moisture loss
Three patches of area 2cm x 2cm were accurately weighed and kept in desiccators for 3 consecutive days. Patches were removed and reweighed. The % moisture loss was calculated using the formula. [32-33]
In vitro dissolution study
The dissolution study of the patch was carried out using modified type 5 dissolution apparatus at 37°C ± 0.5°C using 300 ml of simulated saliva (pH 6.8) as dissolution media. The agitation speed of paddle was 50 rpm. At predetermined time intervals, 5 ml of sample was withdrawn and replaced with fresh medium. The sample was filtered through Whatmann filter paper and analyzed by UV spectrophotometer at 267 nm. [34-35]
RESULT AND DISCUSSION
Melting Point:
The pure drug Ambroxol HCl reported melting point is 2350C while the observed drug's melting point is 2360C. It indicate that the drug in the powder is pure nature and that the powder is sitagliptin phosphate.
Tabel No.4. Melting point of drug

Calibration curve:
The graph of Concentration Vs Absorbance for pure Ambroxol Hydrochloride was found to be in the concentration range of 0.2-1.0 ?g/ml. with the regression coefficient of 0.996.
Tabe No.5. Calibration curve of Ambroxol Hydrochloride


Figure no 12: UV Absorption spectrum of Ambroxol Hydrochloride

Figure No 13: UV absorption spectrum of Ambroxal Hydrochloride in Methanol
The Lambda Max of pure Ambroxol Hydrochloride was found to be 244 nm. It indicate that given sample of drug is pure in nature and it confirmed that given powder is Ambroxol Hydrochloride.
Table No.6.Various Constant for Calibration Curve of Ambroxol Hydrochloride

Fourier Transform Infrared Analysis
Ambroxol Hydrochloride

Figure no 14: FT-IR image of Ambroxol Hydrochloride
Ambroxol Hydrochloride with excipients

Table no 8: Weight variation, Folding endurance, Thickness mucoadhesion time of patch

The weight variation of the patch was found to be in the range of 15.3±0.057 to 28.8±0.173 mg, which meet the criteria as per standard requirement. The thickness of the patches was found to be in the range of 0.25±0.005-0.43±0.002 mm. This may be due to increase in concentration of polymer. Whereas the surface pH of all the formulations was found to be near to salivary pH (5.5±0.057 to 5.6±0.057), this indicates that all the formulations are free from any type of mucosal irritation. The % elongation ranged from 6.21±0.196 to 7.20±0.943, gives an indication about the elasticity of the patch. To find out the flexibility and tensile strength of the patches, folding endurance test and tensile strength test were performed. The result of studies showed that upon increasing the concentration of polymer, the flexibility and tensile strength of the patches increases. This may be due to strong covalent bonding between polymer and drug. The folding endurance found to be in the range of 188.6±0.512 to 262.0±0.05 and tensile strength values ranged from 0.23 - 0.51 Kg/cm2. Drug content of different formulations was found to be in the almost uniform range which indicates that the drug was dispersed uniformly throughout the patch. The value ranged between 85.80% – 95.80%. The % moisture loss of the formulations varied within the range of 2.10±0.100 to 3.48±0.076, which gives an idea about the stability of the patch. The ex vivo residence test performed to determine the ability of patch to retain on mucosa and the values obtained ranged from 320±1.15 to 485±1.00 min, which was found to be satisfactory for all formulations. The drug release profiles of Sitagliptin from formulations F1 to F5 of drug release studies clearly indicate that the drug release was governed by polymer concentration. In the first hr, 40 to 50 % drug was released. This fast release of the drug was due to the erodible, hydrophilic layer of polymer. The hydrophilic polymer HPMC dissolves and creates pores as well as channels for the diffusion of drug from patches. It showed release of 99.7% in 8 hr. Comparing to all other formulations F1(98.9%) in 6 h, F2(62.5%), F3(97.5%) in 6h and F5(75.9 %), F4 proved to be better candidate for releasing 99.7% drug in prolonged period of 8 h.
CONCLUSION:
In conclusion, the mucoadhesive Ambroxol Hydrochloride buccal patch has demonstrated significant promise as an innovative and effective mucolytic treatment. Its sustained drug release, strong mucoadhesive properties, and potential for improved patient compliance make it a viable alternative to conventional mucolytic therapies, offering considerable benefits in the management of respiratory conditions.
REFERENCES
- Prenner BM, Schenkel E. Allergic rhinitis: treatment based on patient profiles. Am J Med. 2006;119(3):230–237. [PubMed] [Google Scholar]
- Shedden A. Impact of nasal congestion on quality of life and work productivity in allergic rhinitis: findings from a large online survey. Treat Respir Med. 2005;4(6):439–446. [PubMed] [Google Scholar]
- Lundbäck B. Epidemiology of rhinitis and asthma. Clin Exp Allergy. 1998;28(Suppl 2):3–10. [PubMed] [Google Scholar]
- Hickner JM, Bartlett JG, Besser RE. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134(6):498–505. [PubMed] [Google Scholar]
- Leggett JE. Acute sinusitis. When – and when not – to prescribe antibiotics. Postgrad Med. 2004;115(1):13–19. [PubMed] [Google Scholar]
- Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–S334. [PubMed] [Google Scholar]
- Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps. Rhinol Suppl. 2007;(Suppl 20):1–136. [PubMed] [Google Scholar]
- Ratner PH, Howland WC, III, Arastu R, et al. Fluticasone propionate aqueous nasal spray provided significantly greater improvement in daytime and nighttime nasal symptoms of seasonal allergic rhinitis compared with montelukast. Ann Allergy Asthma Immunol. 2003;90(5):536–542. [PubMed] [Google Scholar]
- American Academy of Allergy, Asthma and Immunology Available from: http://www.aaaai.org/members/cme_ce/allergicdisorders/case1.stm Accessed August 6, 2009.
- van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55(2):116–134. [PubMed] [Google Scholar]
- Shinde Pramod et al ;" Buccal Film : An innovative Dosage from Designed to Improve Patient Complaince, international journal of pharmaceutical and chemical sciences. 2012;1 (4):12
- Shojaei Amir H., Buccal Mucosa As A Route For Systemic Drug Delivery: A Review, J Pharm PharmaceutSci, 1998;1 (1):15-30
- deVries M.E, Ph.D. Thesis, University of Leiden, Leiden, The Netherlands, 1991
- P.K. Khobrgade et al ;" Literature studies on preparation and evaluation of buccal patches";2014;25(2):73.
- Subhranshu Panda et al ;" Development and Characterisation of muccoadhesive patches of glimipride for buccal administration, journal of pharmaceutical science and bioscientific research"; 2011;1(2): 102-107
- Patil PC, Shrivastava SK, Vaidehi S, Ashwini P. Oral fast dissolving drug delivery system: A modern approach for patient compliance. Int J Drug Reg Affairs. 2014; 2(2): 49-60.
- Kumar RK, Sulochana MM. Fast dissolving films: A unique strategy for drug delivery. Asian J Pharm Res. 2014; 4(1): 47-55.
- Arpita C, Gulab T, Manisha P, Koshy MK, Shubhini AS. Formulation and Charachterization of Carvedilol buccal mucoadhesive patches. Int J Pharm Sci 2010;1(4):396-401.
- Gali AK. Fast dissolving dosage form. Int J Pharm Sci Inv. 2013; 2(11): 14-17.
- Varinder K, Foziyah Z, Geeta A, Ankush C, Formulation and evaluation of buccal patches of Venlafaxine.Int J Pharm Bio Sci 2011;1(3):17-82.
- Patil SL, Mahaparale PR, Shivnikar MA, Tiwari SS, Pawar KV, Sane PN. Fast dissolving oral films: An innovative drug delivery system. Int J Res Rev Pharm Appl Sci. 2(3): 482-96.
- Namita P, Kakar S, Singh R. A review on buccal patches. Innoriginal Int J Sci. 2016;3(5):4-8.
- NB, Sandeep CA, Vikrant PW. A review on buccal drug delivery system. Int J Pharm Pharma Sci.2013;3(1):35-40.
- Vaishali AC, Sarode SM, Sathe BS, Vadnere GP. Mucoadhesive buccal drug delivery system-a review. Int J Pharma Sci.2014;5(2):142-62.
- Mathias NR, Hussain MA. Non-invasive systemic drug delivery: Developability considerations for alternate routes of administration. J Pharm Sci.2010;99(1):318-25.
- Vaishali AC, Sarode SM, Sathe BS, Vadnere GP. Mucoadhesive buccal drug delivery system-a review. Int J Pharma Sci.2014;5(2):142-62.
- Mathias NR, Hussain MA. Non-invasive systemic drug delivery: Developability considerations for alternate routes of administration. J Pharm Sci.2010;99(1):318-25.
- Jyothi A, Gurpreet S, Seema S, Rana AC. Fast dissolving films: A novel approach to oral drug delivery. Int Res J Pharm. 2011; 2(12): 69-74.
- Verma NK, Kumar CS, Prasad H, Srivastava SP, Chandra V. Composition, characterization and application of fast dissolving oral film- A review. Asian J Pharma Tech Innov. 2013; 1(2):1 10.
- Mohasin Lodhi, Akhilesh Dubey, Reema Narayan, Prabhakara Prabhu, Sneh Priya. Formulation and evaluation of buccal film of Ivabradine Hydrochloride for the treatment of stable angina pectoris. Int J Pharm Investig. 2013; 3(1): 47–53.
- Abdelbary A, Bendas ER, Ramadan AA, Mostafa DA. Pharmaceutical and pharmacokinetic evaluation of a novel fast dissolving film formulation of Flupentixol Dihydrochloride. Am Ass Pharm Sci Tech. 2014; 15(6): 1603-10.
- Narendra Nyola. Analytical method development and validation of Sitagliptin phosphate monohydrate in pure and tablet dosage form by derivative spectroscopy.2013;3(1):095-096.
- Poluri K, Mulpur E, Puttugunta SB, Govada KB. Formulation development and evaluation of novel oral soluble films of Ziprasidone hydrochloride in the treatment of schizophrenia. Int J Pharm Pharma Sci. 2013; 5(2): 619-27.
- Sreeja CN, Anoop KR. Design and in vitro evaluation of controlled release Satranidazole subgingival films for periodontitis Therapy. Int J Pharm Sci Rev Res. 2014; 24(1): 8-14.
- Karthikeyan D, Sri S, Kumar CS. Development of fast dissolving oral film containing of Rizatriptan benzoate as an Anti-migraine medication. Indo Am J Pharma Res. 2013; 3(3): 2642 -53.


 Sahil Nandkumar kamble * 1
Sahil Nandkumar kamble * 1
 Radhika S. Subhedar 2
Radhika S. Subhedar 2






















 10.5281/zenodo.13349354
10.5281/zenodo.13349354