Abstract
Over the past three decades, drug delivery management has become more established and plays an important role in drug development. Traditional drug delivery methods have many disadvantages, such as the need to administer specific drugs with short half-lives to maintain the plasma concentration of the drug. Additionally, due to irregular administration, patient compliance is poor, resulting in changes in blood levels. The limitations of traditional drug delivery can be overcome by developing new drug delivery systems that can maintain constant plasma drug levels by slowly releasing the drug over an extended period of time. The development of drug control devices can also improve the bioavailability of drugs, leading to better treatment and better patient compliance. There are many ways to control distribution, such as liposomes, liposomes, ethosomes, phytosomes, microemulsions, and microspheres. Among all the methods, microspheres are easier because the drug is released slowly from the polymer matrix and the polymers used are mostly biodegradable and have no side effects. For this reason, microspheres can be used in many medical fields such as oncology, gynecology, radiology, pulmonology, cardiology, diabetes and medicine. This review article introduces several new types of microspheres and their preparation. The microspheres can then be measured and quantified by different methods.
Keywords
nanoparticles, microspheres, polymers, drug delivery.
Introduction
More than 90% of existing drugs are used in the most popular and easy way: orally. The first challenge faced by pharmaceutical companies when a new drug is discovered is how to create a dosage form that will be effective for oral use in the first place. However, oral drugs suffer from limitations of clinical efficacy due to the need to achieve sustained plasma concentrations and monitor the population. This pharmacokinetic limitation can be overcome by changing the formulation to release the drug slowly upon long-term administration. One of these methods is microspheres, also known as microparticles [1]. Microspheres are characterized by a spherical shape and a free-flowing powder composed mostly of biodegradable polymers. At best, their size varies between 1 µm and 1000 µm. Microspheres can transport drugs and be used for drug delivery. The fact that the drug is loaded into polymer microspheres shows that the treatment is only in the target tissue. Microspheres are designed to enhance the therapeutic effects of drugs and achieve better bioavailability, thereby reducing toxicity and side effects. [2]
There are two types of microspheres: reservoir type and matrix type.
Reservoir type
In this system, the drug acts as a base and is surrounded by a water-insoluble polymer to control the release of the drug. The most commonly used polymers in these materials are ethyl cellulose or polyvinyl acetate. This type is also called microcapsules.
Matrix Type
In this type, the drug is distributed evenly in the polymer matrix, thus controlling the amount of drug released. The most commonly used matrix type polymers are sodium alginate or hydroxypropyl methylcellulose (HPMC). This type is also known as micro matrix [3].
There are two methods for drug release from microspheres
1. Dissolution: Here the dissolution rate is controlled by the polymer decreasing its wet ability or spontaneously dissolving in the intestine at a slow rate.
2. Diffusion: Here, the drug spreads from the high-drug area to the low-drug area [6].
Advantages
Better patient compliance Since microspheres can release the drug slowly over a long period of time, the frequency of drug use is reduced; Since there is a better patient compliance, most drugs are used in children, elderly and psychiatric patients.
Improved Bioavailability Microsphere size is in the micron range, meaning the small size provides a large surface area to increase the solubility of poorly soluble drugs, thus improving the systemic bioavailability of drugs. Stable plasma drug concentration
Microspheres show prolonged release of the drug; Therefore, the drug concentration in the body does not change and reaches a constant Cmax value.
Reduce Adverse Effects Biodegradable polymer microspheres are biocompatible with the body's environment; They do not require surgical removal. Since the drug is released in a controlled manner, its toxicity is reduced. Improve stability
Liquid medicine can be converted into microspheres to ensure the stability of the medicine and maintain its clinical life. < br>
Parenteral preparations The shape of the microspheres is spherical and the amount of drug can be based on parenteral deposits of the microsphere. Targeted Drug Delivery
Microspheres are used to target diseases, particularly tumors, where the concentration in the rest of the tissue is low [4,5].
Disadvantages
Cost of production - The cost of production of prescription drugs is higher than the cost of production of prescription drugs.
Repeatability - Microspheres are difficult to reproduce because they require special techniques and technology.
Potential Toxicity - Since microspheres contain large amounts of drug, chemical waste will be generated, leading to potential toxicity.
Polymer Toxicity - Polymer additives such as plasticizers, stabilizers and antioxidants are also potentially toxic. Depending on their structure, these polymers can hydrolyze, oxidize, or react with biological substances, causing toxicity.
Swallowing - Microspheres used for oral administration should be swallowed rather than chewed or crushed because they are designed to delay the release of the drug.
Healing - conditions The performance of microspheres such as pH, temperature, mixing, solvent evaporation and heat will affect the stability of the drug to be encapsulated [4, 5].
TYPES OF MICROSPHERES
Bio adhesive Microspheres
Drug-loaded microspheres can be applied to eyes, anus, nose, etc. It uses the adhesive properties of water-soluble substances to adhere to the polymers of mucous membranes. This is called bio adhesion. This type of microspheres exhibit properties such as being close to the absorption surface. It has also been shown to have a longer residence time at the target site and lead to better results, such as facial application of acyclovir on bio adhesive microspheres, nasal application of insulin, and buccal application of nifedipine [7,8].
Magnetic Microspheres
Such microspheres have the important feature of being used for distribution purposes, for example, limiting the drug to the disease area. The main purpose of this type of microspheres is to replace large doses of free diffusion with small doses of magnetically focused drugs. Since the size of magnetic microspheres is <4>
There are two types of magnetic microspheres: Therapeutic magnetic microspheres These types of microspheres generally target breast cancer by delivering chemotherapy drugs to the target area, namely pain. Such microspheres are usually filled with protein or peptide drugs for targeting.
Diagnostic Magnetic Microspheres
These microspheres are used only to evaluate liver metastases. It can also be used to make nano-sized materials such as super magnetic iron oxide, which can be used to differentiate intestines from other intestinal diseases.
Floating Microspheres
Floating microspheres have a smaller volume, so they float in the stomach without affecting the digestive system. During the slow release of the drug on demand, such microspheres have been shown to affect the digestive system and increase the residence time in the stomach, leading to a constant plasma concentration. As a result, the control frequency of these microspheres is also reduced, and long-term therapeutic effects such as ketoprofen floating microspheres and felodipine floating microspheres are also produced [11,12].
There are two types of floating microspheres:
- Effervescent Microspheres
- Non-effervescent microspheres
Radioactive microspheres
Radioactive microspheres The size of radioembolization microspheres is 10-30 nm; this is larger than the capillary diameter and hits the first capillary bed. Microspheres are injected into blood vessels leading to the target tumor. Therefore, in all of these, radio microspheres do not harm the rest of the tissue, they concentrate in certain areas and emit high radiation. Different types of radioactive microspheres are alpha emitters, beta emitters and gamma emitters [13].
Polymer Microspheres
Various types of polymer microspheres can be classified as follows.
Biodegradable Polymer Microspheres
Starch used in microsphere formulation is a natural polymer; It is biodegradable, biocompatible and bio adhesive in nature. Such biodegradable polymers can last for a long time and eventually form a gel when in contact with the mucosa due to their swelling properties due to contact with aqueous media. The quantity and amount of drug released from the microspheres depends entirely on and is controlled by the concentration and release pattern of the polymer. This release pattern occurs continuously. The main disadvantage of biodegradable microspheres is drug loading efficiency and drug release. However, they are widely used in microsphere-based therapy; for example, polylactic acid microspheres loaded with 5-fluorouracil.
Synthetic polymer microspheres
such microspheres are widely used in medical applications. It is also used as skin tightening agents, fillers, embolic agents, drug carrier agents, etc. They can also be used and proven. are safe, biocompatible, but the main disadvantage of such microspheres is that they can migrate from the injection site and cause risk, embolism and other organ damage, for example, phenobarbital microspheres using the Eudragit RL polymer [14]
Porous Microspheres
Porous microspheres have two external surfaces or a significant number of internal pores through which active pharmaceutical ingredients can disperse or dissolve as directed. 1. Pores are formed by porogens that are completely dissolved during the process; For example, porogens used are ammonium bicarbonate, effervescent salts such as hydrocarbon waxes, inorganic salts such as sodium chloride, carbohydrates, ice, linear polymers, gelatin and sugar. The coating system is made of materials such as calcium carbonate (CaCO3), mesoporous silica, hydroxyapatite and biodegradable porous starch foam used in the transport of proteins and peptides [15,16].
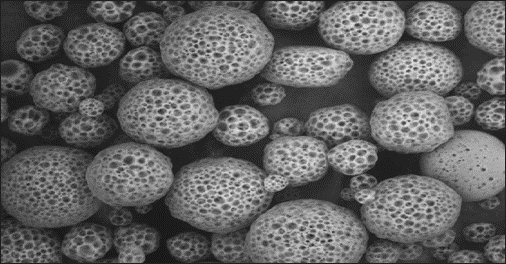
Fig. 1: Porous microspheres as observed under the scanning electron microscope. [17]
MICROSPHERE INGREDIENTS [18]
Polymers
In microsphere formulations, researchers often use a variety of biodegradable and non-biodegradable polymers. Polymers used in the preparation of microspheres are divided into two: natural and synthetic. Before selecting the polymer for the production of microsphere, we should consider some non-toxicity, biocompatibility, biodegradability and simplicity of the polymer. It must be biocompatible, biodegradable, non-toxic and easily available. Polymers that pass all the options do not have many advantages, such as increasing the time the drug stays in the body and thus providing better bioavailability of the drug compared to the drug formula. Examples of natural polymers include albumin, collagen, and gelatin, which are proteins, while agarose, carrageenan, chitosan, and starch are carbohydrates, and poly(acrylic acid) dextran, polystarch, and DEAE cellulose are carbohydrates chemically treated with sodium. alginate, cellulose ethers, xanthan gum, Scheroglucan, arabica gum, tamarind seed polysaccharide, beeswax, carnauba wax, chitin and zein (Zien). Examples of synthetic polymers are divided into three groups: lactide, glycolide and their copolymers, polyanhydrides, polyalkyl cyanoacrylates are biodegradable in nature, while glycidyl methacrylate, propylene Aldehydes, epoxy polymers and polymethyl methacrylate’s are a polyhydrate, ultimately a polyhydrate. /polylactide, polyorthoesters, polycarbonate, polylactic glycolic acid (PLGA), Polycaprolactone, polyphosphazen, ethyl cellulose, Eudragit L100, Eudragit S100, HPMC, Eudragit RS100 and Eudragit RL100 are another group [19-24].
Surfactants
Surfactants play an important role in the emulsification and extrusion process in microsphere formation. Surfactants play an important role by reducing the interaction between hydrophilic and hydrophobic molecules, thus forming a stable emulsion. The use of surfactants prevents the emulsion droplets from coalescing to form separate microspheres. The hydrophile-lipophile balance (HLB) indicator is used to select suitable emulsifiers. While hydrophilic surfactants with HLB values ??in the range of 8-18 are used in oil-in-water emulsions, emulsifiers with HLB values ??in the range of 3.5-6 are called lipophilic surfactants. As the surfactant concentration increases, the particle size of the microspheres decreases and a small and particle size distribution occurs. For example, sodium laureth sulfate, sodium lauryl sulfate as anionic surfactants, as well as polysorbate 80, Tween 40, Tween 20, Span 85, Span 80, Span 20, Poloxamer 188, Brij58, polyglycerol, sorbitol and sorbitol-free polyricinoleate.[31].
Oil
The particle size, distribution and uniformity of microspheres are affected by the effect of the ratio of the viscosity of the oil phase to the viscosity of the aqueous phase, for example. It has been reported that the particle size of microspheres prepared using olive oil is larger than that of microspheres prepared using liquid paraffin, since the viscosity of olive oil is higher than liquid paraffin. There are different types of oils used to create microspheres during the emulsification/gel process. Examples include liquid paraffin, soybean oil, olive oil, sunflower oil, castor oil, peanut oil, rapeseed oil and rapeseed methyl ester [32].
Crosslinking agents
The most commonly used crosslinking agents in microsphere preparation are Ca2+, Sr2+ and Ba2+ ions. However, Sr2+ and Ba2+ ions are slightly toxic, while Ca2+ ions are non-toxic, so Ca2+ ions are widely used as crosslinkers in the preparation of microspheres. At a low Ca2+ ion concentration, the microspheres aggregate. By increasing the Ca 2+ ion concentration, the encapsulation efficiency of the microspheres increased slightly. However, after the cross-linker reaches the optimum concentration, if there is more cross-linker, the performance of the product will decrease due to cross-linking of too many links. Examples include glutaraldehyde, sulfuric acid, and calcium carbonate [33–36].
Solvents
Solvents are commonly used when preparing microspheres by solvent evaporation. Examples include chloroform, dichloromethane (DCM), ethanol, acetonitrile, polyvinyl alcohol (PVA), dichloromethane and methanol [37–39].
Dry Method
In this technology, the polymer layer is first dissolved/dissolved in organic solvents such as acetone and DCM and then the solution is placed into the polymer by high-speed homogenization [40]. The resulting mixture is then atomized in a stream of hot air. Atomization occurs through the formation of fine air or droplets and the organic solvent immediately evaporates, resulting in the formation of microspheres of size 10 ?m-100 ?m [41].
Solvent evaporation
This method uses organic processes as the means of production; This process has two stages. The first is the aqueous phase in which the drug is incorporated with or without stabilizers. Moreover, the other phase is the organic phase containing the polymer in organic solvents such as acetone and DCM, then it is aqueous and the organic phase needs to be mixed by high-speed homogenization, which forms w/o emulsion. This emulsion is added. If necessary, add to the aqueous phase to form a water/water/water emulsion. The mixture is heated with constant stirring, causing the organic phase to evaporate, causing the polymer layer of the main product to shrink and microspheres to form.
Single emulsion technology
This method uses emulsification technology to prepare microspheres; The polymer layer is dissolved in a heavy organic solvent to form a polymer solution. Addition of the resulting polymer to the aqueous phase containing the emulsifier forms an O/W emulsion. The emulsion is then stirred for several hours under constant ambient conditions, filtered and dried in a desiccator [42].
Double Emulsion Technology
This technology involves the preparation of double emulsion by two methods: /o/w or o/w/o mode. Aqueous solutions contain chemicals dispersed in the organic phase. The organic phase with a polymer layer encapsulates the drug present in the dispersed aqueous phase, resulting in the formation of a primary emulsion. This first emulsion is then homogenized or ultrasonicated and then added to PVA aqueous solution to form a second emulsion, which is then filtered and dried in a desiccator to prepare microspheres [43].
Phase separation coacervation technology
This is generally used to prepare reservoir type microspheres. This method is often used to encapsulate hydrophilic drugs; In this way, the polymer layer is dissolved in an organic volatile solvent, and then an aqueous solution of the drug is added to coat the drug with the polymer, and then the drug is coated with it. polymer by changing the environment (such as changing temperature, changing pH, and adding salt)
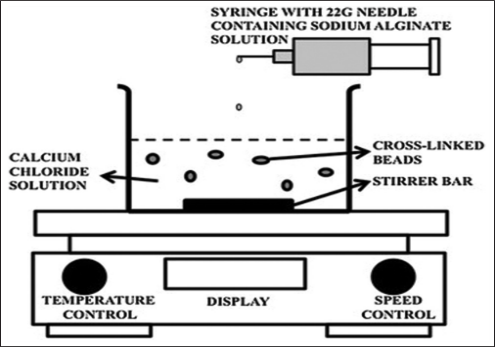
Fig. 2: Schematic illustration of the ionic gelation method. [30]
Spray Coagulation
In this process, the drug is dissolved/dispersed in a polymer solution, for example, a lipophilic polymer such as wax. The hot molten solution is then sprayed into the well water in the container, which is stored in ice water carbon dioxide [45].
Liquid Extraction
This method removes the organic phase by extracting the organic phase. Solventization is accomplished using a hydrophilic organic solvent such as isopropyl alcohol. The organic phase is then extracted with water; this is a process that shortens the hardening time of microspheres [46].
Semi-emulsion solvent diffusion
Micro sponges can be prepared using this technique. It has two levels, one inside and the other outside. The outer phase contains PVA and distilled water, while the inner phase contains polymer, chemicals and ethanol. Preheat the interior to 60°C and add the bones to the outside. Then store at room temperature. The resulting emulsion was then homogenized for 2 h to form micro sponges, which were filtered, washed and dried in a vacuum oven for 24 h [47].
Crosslinker method
In this method, a crosslinker - binding agent is used in the production of microspheres. The first special polymer solution is prepared in an aqueous medium and then added to the continuous phase containing oil and a certain surfactant content to form a w/o emulsion, then the aqueous solution of the cross-linker is combined dropwise with the continuous phase. mix and then harden the surface of the microspheres. The resulting microspheres were washed and dried [48].
Hot Melt Microcapsules
In this technology, the polymer layer is melted and homogenized with the drug, and the resulting mixture is suspended in a lipophilic solvent (e.g. silicone) by adding additional oil and continues. The solution was heated to the melting point of the polymer at 5 °C with stirring/stirring and after stabilizing the emulsion, it was cooled to solidify the polymer microspheres [49].
Ion Gel Method
In this method, a suspension of hydrophilic polymers and chemicals is complexed with multivalent cations (e.g. calcium chloride) to form viscous gel spheres as shown in Figure 1. 2. Get an iridescent suspension. This suspension was centrifuged to obtain small-sized microspheres. The microspheres were then washed and dried at room temperature for 24 h [50,51].
Hydroxy Appetite (HAP) Microspheres in Spherical Morphology Microspheres were prepared from o/w emulsion after evaporation of the organic solvent. First organic phase (contains 5% chemicals by weight)
STUDY TECHNIQUES
Characterization
Characterization of these special transporters is an important phenomenon that helps in the creation of high protein and suitable foods, drugs or antibiotics. Each microsphere has a different microstructure. These microstructures determine the release and stability of the carrier.
Particle size and shape
The most commonly used microsphere imaging methods are normal light microscopy (LM), confocal fluorescence microscopy and scanning electron microscopy (SEM). These techniques can be used to determine the shape and external structure of microspheres. Pa LM: Pa LM provides process control in the case of double-walled microspheres. The structure of the microspheres can be seen before and after coating, and the changes can be measured with a microscope. SEM provides higher resolution compared to LM. SEM: SEM can examine the surface of microspheres and can also be used to examine double-walled systems after sectioning the material [59]. Confocal fluorescence microscopy: Confocal fluorescence microscopy is used to pattern multi-walled microspheres. In addition to measurement techniques, laser light scattering and multiple Coulter counters can be used to characterize the size, shape, and morphology of microspheres [55].
Angle
Let the contact angle determine the hydrophobic and hydrophilic wetting properties of the microspheres. This thermodynamic property is specific to solid matter and is affected by the presence of absorbent material. The contact angle is measured at the material/air/water interface. The increase and decrease in contact angle is measured by placing the drop in the circle on the circle mounted above the target of the inverse measuring device. Contact angles were measured at one minute of microsphere deposition at 200°C [57].
Attenuated Total Reflection Fourier Transform Infrared (FT-IR) Spectroscopy
FT-IR determines the degradation of the microsphere polymer matrix by calculating the alternating total reflectance (ATR) for the reinforcement and the microsphere surface. A beam of infrared light passes through the ATR crystal and is reflected many times inside the sample, giving the infrared spectrum of most material surfaces. ATR-FTIR integration provides information about the surface of sample microspheres [57].
Density Determination
Multi-volume pycnometers are used to determine the density of microspheres. Place the correct microsphere sample into the beaker, then place the beaker into the multi-volume pycnometer. Place the glass in a multi-volume pycnometer. Under constant pressure, helium gas is introduced into the chamber and allowed to expand. The expansion of the helium reduces the pressure in the chamber and two consecutive readings of the decrease in the first difference are recorded. From these two pressure readings, the volume and density of the sample microspheres can be determined [57].
Electron Spectroscopy for Chemical Analysis (ESCA)
ESCA determines the surface chemistry of microspheres. ESCA also uses electron spectroscopy to produce spectra to determine the atomic composition of the microsphere surface and the degradation of biodegradable microspheres. It is also called X-ray photoelectron spectroscopy [57].
Surface Carboxylic Acid Residues
Radioactive glycine is used to measure surface carboxylic acid residues. Radioactive glycine conjugates are synthesized by the reaction of c14-glycine ethyl ester hydrochloride and sample microspheres. Glycine residues are formed using the water-soluble condensate 1-ethyl-3 (3-dimethylaminopropyl) carbodiimide (EDAC). The radioactivity of the conjugates was measured using liquid scintillation counter technology. Therefore, carboxylic acid residues can be compared with the structure and the requirements can be drawn accordingly. Free carboxylic acid residues can be used to measure hydrophobicity or hydrophilicity or other types of derived microspheres [57]. Isoelectric Point
To determine the isoelectric point, a device called microelectrophoresis is used to measure the electrophoretic movement of microspheres. The average velocity for each pH value in the range 3 to 10 was calculated by measuring the time it took for the object to travel a distance of 1 mm. This information can be used to determine the electrical conductivity of the material. The electrophoretic movement can be related to three parameters such as surface charge, ionization behavior or ion absorption properties of microspheres [57].
Tree Amino Acid Residues
Radioactive c14-acetic acid conjugates are used to locate amino acid residues. Amino acid residues were first determined indirectly by measuring the carboxylic acid residue with a liquid scintillation counter. EDAC is used to condense amino and c14-acetic acid carboxylic acid residues. Indirect estimation of free amino or free carboxylic acid residues by measuring the radioactivity of C14 glycine ethyl ester hydrochloride with acetic acid or glycine conjugates [57].
Capture Efficiency
The capture efficiency of microspheres is determined by washing the microspheres in lysis buffer. The lysate is then tested as specified in the specific monograph to calculate the content of the preparation. The percentage of encapsulation efficiency was calculated using the following formula: Percentage of encapsulation efficiency = actual content/theoretical content × 100 [57].
In Vivo Methods
In vivo methods are ways to study the permeability of the mucosa. This technique uses the biological response of local organisms or the body. Some of the earliest and simplest studies of mucosal permeability were determined by the pharmacological effects of drugs upon ingestion or inhalation into the oral mucosa. However, currently the most commonly used methods are examining drug permeability using animal models, oral tests, and bone perfusion chambers [4].
Animal Models
A number of compounds have been studied using animal models to study drug permeability. Review the process and use of access tools or testing methods. Many animal models are known, such as dogs, rats, rabbits, cats, hamsters, pigs and sheep. This procedure involves anesthetizing the animal and then giving it a large amount of paper to examine. The esophagus of rats is ligated to prevent absorption through the oral mucosal layer. The absorption rate is determined by taking blood from different points and analyzing.
Buccal Absorption Test
It is known for its simplicity and reliability by measuring the absorption of drugs in the mouth. Single and multiple drug combinations. Thanks to this test, the structure of the drug in the oral cavity, contact time, pH and initial drug concentration can be determined [61].
Core Perfusion Chamber
In Development The corneal perfusion chamber method will be very useful compared to the evaluation of ophthalmic drugs. The aim of this study is to design and test a modified perfusion chamber suitable for topical application of drugs isolated from the corneoscleral process and allowing continuous monitoring of the function of endothelial cells. In this method, the perfusion chamber is made of polycarbonate and stainless steel and clamps the cornea to a horizontal surface, making it suitable for local drug delivery. During this perfusion, endothelial cell function was assessed by ultrasound pachymetry and specular microscopy. Epithelial barrier function was assessed by luciferin penetration. Analyze flow by measuring protein penetration. After perfusion, tissues were examined by routine histology [56].
In Vitro Method
Beaker Method In this method, the dosage form is adhered to the bottom of a beaker with a medium and mixed thoroughly using a head. The medium used is 50 to 500 ml and the stirrer speed is 60 to 300 rpm. The sample is removed in a short time and the solution in the environment is determined [60].
Interface Distribution System
Interface Distribution System was developed by Dearden & Tomlinson. It has four compartments. Compartment A represents the oral cavity containing the appropriate concentration of allergen. Column B represents bacterial membrane containing 1-octanol, and column C represents body fluid containing 0.2 M HCl. Compartment D represents protein binding and also contains 1-octanol. Before use, the aqueous phase and 1-octanol were saturated together. Remove the sample with the syringe and return to area A. Therefore, the drug dissolved in different parts of the human body is determined by analyzing samples from four parts [4].
Dissolution Apparatus
A standard USP or BP dissolution apparatus is used to examine in vitro drug release profiles using two components: spoon 41, 42, 43 and basket 44, 45. It varies between 500 ml and the rotation speed varies between 50 and 100 rpm [60].
Other Methods
Several other methods of placing a sample on Perspex in a glass (46), agar gel method (47), etc. has also been reported. Although many methods have been reported, the best method is one of tank conditions and in vitro relaxation times that results in in vivo relaxation times [4].
Use of Microspheres in the Pharmaceutical Industry
Oral Drug Delivery
Oral route is a simple and easy way of administration with patient compliance and personal care. Many medications are administered orally. The principle of oral absorption depends on the solubility and permeability of the drug. Micro spherical drug delivery provides drug resistance and controlled release, which can reduce the frequency of drug use and improve long-term patient compliance [62].
Ocular Drug Delivery
Microspheres are good carriers for drug delivery to the eye. Drug bioavailability is improved by micro spherical drug delivery compared to aqueous ocular formulations. Due to their controlled release mechanism, microspheres can be used for long-term drug delivery, thus reducing the frequency of drug administration [63].
Intranasal Drug Delivery
This method is used to deliver proteins and peptides. The prepared air easily disappears from the nasal mucosa. Bio adhesive microspheres provide better bioavailability using their risk/control [64].
Gene Therapy
In this technology, microspheres are created to use bacterial cells to provide gene therapy. Compared to direct vector delivery, this device is easy to prepare, localize and produce at scale and shows a lower immunogenic response [65].
Oral drug delivery
Microspheres adhering to the mucosa as a reservoir for drugs; may leave the drug at the application site for a long time. Mucoadhesive polymers in the buccal mucosa act as reservoirs; It also increases the bioavailability of the drug by preventing first-pass metabolism in the body [62].
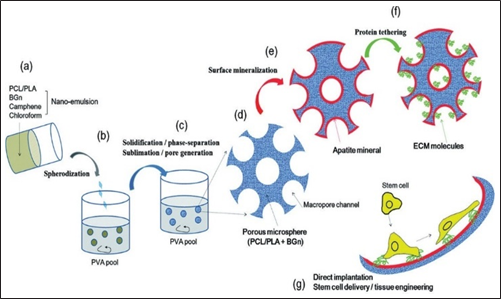
Fig. 3: Microspheres application in bone tissue engineering. Adapted from reference [56]
Transdermal and Topical Drug Delivery
Polymers with good film-forming ability such as chitosan, alginate are used for transdermal delivery of drugs and PLGA loaded microspheres are used for transdermal drug delivery. It is also used to deliver topical agents such as asiaticoside-loaded microspheres for wound healing; where it has been shown to accelerate epithelial regeneration and promote angiogenesis [66].
Gastrointestinal Drug Delivery
Microspheres are used to effectively deliver drugs to a specific site (gastrointestinal tract [GIT]).
Eudragit, ethyl cellulose, carbomer and alginate microspheres are used to deliver drugs to specific areas in the GIT. It blocks the first-pass hepatic metabolism of drugs and increases their bioavailability [67].
Intratumoral and local delivery
Antitumor drug must be delivered to the tumor site in an inappropriate manner, such as paclitaxel-loaded microspheres. Film-forming polymers are used to control release at local sites (e.g. oral cavity) [68].
Colon Drug Delivery
Microspheres are used to deliver medication to a part of the colon, namely the colon. Insulin was encapsulated in chitosan microspheres to release the drug in the intestine [69].
Vaginal
Birth Microsphere delivery is used to treat vaginal infections such as vaginal yeast infections. Chitosan, gelatin and PLGA polymers are used to produce microspheres for the treatment of genital warts [70].
Radioactive Applications
Radioactive isotopes of elements have been used in medicine for many years [71-83] Isotope-loaded microspheres, such as yttrium, are used in the treatment of many diseases such as liver and spleen cancer. 90. Packet of charged microspheres is used to treat cancer [84].
Injectable drug delivery
Microsphere delivery has been shown to have the best drug response; Thiolate Eudragit microspheres may be a better candidate for oral contraceptives. It can affect the immune system and mucosa [57].
Targeting uses most drugs
Targeting means that the response caused by the drug depends on receptor access and molecular interaction. Most techniques are used to target specific organs or receptors. Microspheres can be prepared by extrusion/spheronization techniques such as chitosan and microcrystalline cellulose [57].
Application in Dentistry
Microspheres are used in dental preparations to treat various oral diseases such as gingivitis and bleeding gums. Microspheres are also used for craniofacial tissue regeneration [85,87–89]. Applications in bone tissue engineering
Microspheres can be used as stable cells in tissue engineering to deliver MSC (bone marrow mesenchymal stem cells) leading to rapid bone formation and bone regeneration [86, 90] Ben. The process is shown in Figure 3.
CONCLUSION
Microspheres are a new type of drug delivery in which drugs are packaged into spherical structures composed of polymers, the difference forming the matrix. The drug is slowly released from the microspheres through the matrix system. Different types of microspheres can be used depending on the type of polymer used, and each type of microsphere has its own unique properties. There are also various methods prepared for the synthesis of microspheres. Microspheres are versatile and have a wide range of applications, from diagnostics to drug delivery and therapeutic applications.
REFERENCE
- Kakar S, Jain A. Magnetic microspheres: An Overview. Asian Pac J Health Sci 2019;6:81-9.
- Sharma M, Dev SK, Kumar M, Shukla AK. Microspheres as a suitable drug carrier in sustained release drug delivery: An overview. Asian J Pharm Pharmacol 2018;4:102-8.
- Nidhi P, Anamika C, Twinkle S, Mehul S, Hitesh J, Umesh U. Controlled drug delivery system: A review. Indo Am J Pharm Sci 2016;3:227-33.
- Prasad BS, Gupta VR, Devanna N, Jayasurya K. Microspheres as drug delivery system-a review. J Glob Trends Pharm Sci 2014;5:1961-72.
- Virmani T, Gupta J. Pharmaceutical application of microspheres: An approach for the treatment of various diseases. Int J Pharm Sci Res 2017;8:3252-60.
- Lengyel M, Kállai-Szabó N, Antal V, Laki AJ, Antal I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci Pharm 2019;87:20.
- Farraj NF, Johansen BR, Davis SS, Illum L. Nasal administration of insulin using bioadhesive microspheres as a delivery system. J Control Release 1990;13:253-61.
- Genta I, Conti B, Perugini P, Pavanetto F, Spadaro A, Puglisi G. Bioadhesive microspheres for ophthalmic administration of acyclovir. J Pharm Pharmacol 1997;49:737-42.
- Chandna A, Batra D, Kakar S, Singh R. A review on target drug delivery: Magnetic microspheres. J Acute Dis 2013;2:189-95.
- Zhang J, Zhang S, Wang Y, Zeng J. Composite magnetic microspheres: Preparation and characterization. J Magn Magn Mater 2007;309:197-201.
- Sangale SB, Barhate SD, Jain BV, Potdar M. Formulation and evaluation of floating felodipine microsphere. Int J Pharm Res Dev 2011;3:163-70.
- Srivastava AK, Ridhurkar DN, Wadhwa S. Floating microspheres of cimetidine: Formulation, characterization and in vitro evaluation. Acta Pharm 2005;55:277-85.
- De Cuyper M, Bulte JW, editors. Urs HÄfeli. In: Radioactive Microspheres for Medical Applications. Physics and Chemistry Basis of Biotechnology. Vol. 7. Springer: Dordrecht; 2001. p. 213-48.
- El-Helw AM, Al-Hazimi AM, Youssef RM. Preparation of sustained release phenobarbitone microspheres using natural and synthetic polymers. Med Sci 2008;15:39-51.
- Cai Y, Chen Y, Hong X, Liu Z, Yuan W. Porous microsphere and its applications. Int J Nanomed 2013;8:1111.
- Zhang CZ, Niu J, Chong YS, Huang YF, Chu Y, Xie SY, et al. Porous microspheres as promising vehicles for the topical delivery of poorly soluble asiaticoside accelerate wound healing and inhibit scar formation in vitro and in vivo. Eur J Pharm Biopharm 2016;109:1-3.
- Budov VV. Hollow glass microspheres. Use, properties, and technology. Glass Ceram 1994;51:230-5.
- Li S, Nguyen L, Xiong H, Wang M, Hu TC, She JX, et al. Porous- wall hollow glass microspheres as novel potential nanocarriers for biomedical applications. Nanomed Nanotechnol 2010;6:127-36.
- Ratnaparkhi M, Wattamwar M, Jadhav A, Chaudhari S. Mucoadhesive microsphere-review. Int J Drug Dev Res 2014;6:975-1344.
- Degim IT, Çelebi N. Controlled delivery of peptides and proteins. Curr Pharm Des 2007;13:99-117.
- Bansal H, Kaur S, Gupta A. Microsphere: Methods of prepration and applications; a comparative study. Int J Pharm Sci Rev Res 2011;10:69-78.
- Wu L, Wang M, Singh V, Li H, Guo Z, Gui S, et al. Three-dimensional distribution of surfactant in microspheres revealed by synchrotron radiation X-ray microcomputed tomography. Asian J Pharm Sci 2017;12:326-34.
- Avachat A, Bornare P, Dash R. Sustained release microspheres of ropinirole hydrochloride: Effect of process parameters. Acta Pharm 2011;61:363-76.
- Ranjha NM, Khan H, Naseem S. Encapsulation and characterization of controlled release flurbiprofen loaded microspheres using beeswax as an encapsulating agent. J Mater Sci Mater Med 2010;21:1621-30.
- Pachuau L, Mazumder B. A study on the effects of different surfactants on ethyl cellulose microspheres. Int J Pharm Tech Res 2009;1:966-71.
- Kim JC, Song ME, Lee EJ, Park SK, Rang MJ, Ahn HJ. Preparation of microspheres by an emulsification-complexation method. J Colloid Interface Sci 2002;248:1-4.
- De Rosa G, Iommelli R, La Rotonda MI, Miro A, Quaglia F. Influence of the co-encapsulation of different non-ionic surfactants on the properties of PLGA insulin-loaded microspheres. J Control Release 2000;69:283-95.
- Gaur PK, Mishra S, Bajpai M. Formulation and evaluation of controlled- release of telmisartan microspheres: In vitro/in vivo study. J Food Drug Anal 2014;22:542-8.
- Dinarvand R, Moghadam SH, Sheikhi A, Atyabi F. Effect of surfactant HLB and different formulation variables on the properties of poly-D, L-lactide microspheres of naltrexone prepared by double emulsion technique. J Microencapsul 2005;22:139-51.
- Shiga K, Muramatsu N, Kondo T. Preparation of poly (D, L?lactide) and copoly (lactide?glycolide) microspheres of uniform size. J Pharm Pharmacol 1996;48:891-5.
- Wan LS, Heng PW, Chan LW. Surfactant effects on alginate microspheres. Int J Pharm 1994;103:267-75.
- Kumbar SG, Kulkarni AR, Aminabhavi TM. Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: Effect of crosslinking agent. J Microencapsul 2002;19:173-80.
- Gülsu A, Ayhan H, Ayhan F. Preparation and characterization of ketoprofen loaded albumin microspheres. Turk J Biochem 2012;37:120-8.
- Jayan SC, Sandeep AV, Rifash M, Mareema CM, Shamseera S. Design and in vitro evaluation of gelatin microspheres of salbutamol sulphate. Hygeia 2009;1:17-20.
- Wei W, Wang LY, Yuan L, Wei Q, Yang XD, Su ZG, Ma GH. Preparation and application of novel microspheres possessing autofluorescent properties. Adv Func Mater 2007; 17:3153-8.
- Behera AL, Patil SV, Sahoo SK. Formulation and characteristics of 5 fluorouracil microspheres by solvent evaporation method. Int J Pharm Pharm Sci 2011;3:32-5
- Kendre P, Chaudhari P. Formulation and evaluation of telmisartan microspheres by solvent evaporation technique. Indo Am J Pharm Res 2012;2:651-7.
- Uyen NT, Hamid ZA, Tram NX, Ahmad N. Fabrication of alginate microspheres for drug delivery: A review. Int J Biol Macromol 2020;153:1035-46.
- Uyen NT, Hamid ZA, Ahmad NB. Synthesis and characterization of curcumin loaded alginate microspheres for drug delivery. J Drug Deliv Sci Technol 2020;58:101796.
- Mua L, Fenga SS. Fabrication, characterization and in vitro release of paclitaxel (TaxolÒ) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release 2001;76:239-54.
- Zalloum NL, de Souza GA, Martins TD. Single-emulsion P (HB-HV) microsphere preparation tuned by copolymer molar mass and additive interaction. ACS Omega 2019;4:8122-35.
- Yanga YY, Chiab HH, Chunga TS. Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/ evaporation method. J Control Release 2000;69:81-96.
- Bhattacharya S, Alam M, Dhungana K, Yadav S, Chaudhary KR, Chaturvedi KK, et al. Preparation and evaluation of diclofenac gelatin microspheres using coacervation technique. Int J Pharm Res Innov 2020;13:14-21.
- Bertoni S, Albertini B, Passerini N. Different BCS Class II drug- gelucire solid dispersions prepared by spray congealing: Evaluation of solid state properties and in vitro performances. Pharmaceutics 2020;12:548.
- Gurung BD, Kakar S. An overview on microspheres. Int J Health Clin Res 2020;3:11-24.
- Baimark Y, Srisuwan Y. Preparation of polysaccharide-based microspheres by a water-in-oil emulsion solvent diffusion method for drug carriers. Int J Polym Sci 2013;2013:1-6.
- Kim JU, Shahbaz HM, Lee H, Kim T, Yang K, Roh YH, et al. Optimization of phytic acid-crosslinked chitosan microspheres for oral insulin delivery using response surface methodology. Int J Pharm 2020;588:119736.
- Mathiowitz E, Langer R. Polyanhydride microspheres as drug carriers I. Hot-melt microencapsulation. J Control Release 1987;5:13-22.
- Khanam N, Alam MI, Sachan AK, Gangwar SS. Fabrication and evaluation of propranolol hydrochloride loaded microspheres by ionic- gelation technique. Pharm Lett 2012;4:815-20.
- Patel N, Lalwani D, Gollmer S, Injeti E, Sari Y, Nesamony J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog Biomater 2016;5:117-33.
- Fujii S, Okada M, Sawa H, Furuzono T, Nakamura Y. Hydroxyapatite nanoparticles as particulate emulsifier: Fabrication of hydroxyapatite- coated biodegradable microspheres. Langmuir 2009;25:9759-66.
- Trivedi P, Verma AM, Garud N. Preparation and characterization of aceclofenac microspheres. Asian J Pharm 2014;2:110-5.
- Pradeesh TS, Sunny MC, Varma HK, Ramesh P. Preparation of microstructured hydroxyapatite microspheres using oil in water emulsions. Bull Mater Sci 2005;28:383-90.
- Naveen HP, Nesalin JA, Mani TT. A modern review on microsphere as novel controlled drug delivery system. Asian J Res Pharm Sci Biotechnol 2014;2:62-9.
- Thiel MA, Morlet N, Schulz D, Edelhauser HF, Dart JK, Coster DJ, et al. A simple corneal perfusion chamber for drug penetration and toxicity studies. Br J Ophthalmol 2001;85:450-3.
- Rastogi V, Shukla SS, Singh R, Lal N, Yadav P. Microspheres: A promising drug carrier. J Drug Deliv Ther 2016;6:18-26
- Remuñán-López C, Portero A, Vila-Jato JL, Alonso MJ. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayeredadevices for buccal drug delivery. J Control Release 1998;55:143-52.
- Masaeli R, Kashi TS, Dinarv R, Tahriri M, Rakhshan V, Esfandyari- Manesh M. Preparation, characterization and evaluation of drug release properties of simvastatin-loaded PLGA microspheres. Iran J Pharm Res 2016;15:205-11.
- Tejash P, Shah CN, Shah DP. Microspheres: As a novel controlled drug delivery system a review. Pharm Sci Monit 2016;7:37-53.
- Rathbone MJ. Human buccal absorption. I. A method for estimating the transfer kinetics of drugs across the human buccal membrane. Int J Pharm 1991;69:103-8.
- Jiang WZ, Cai Y, Li HY. Chitosan-based spray-dried mucoadhesive microspheres for sustained oromucosal drug delivery. Powder Technol 2017;312:124-32.
- Liu W, Lee BS, Mieler WF, Kang-Mieler JJ. Biodegradable microsphere- hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr Eye Res 2019;44:264-74.
- Sahu Y, Jain S, Shukla K. Mucoadhesive microspheres based formulation development of ziprasidone hydrochloride for nasal delivery. J Drug Deliv Ther 2020;10:175-81.
- Xu Q, Leong J, Chua QY, Chi YT, Chow PK, Pack DW, et al. Combined modality doxorubicin-based chemotherapy and chitosan-mediated p53 gene therapy using double-walled microspheres for treatment of human hepatocellular carcinoma. Biomaterials 2013;34:5149-62.
- Lee S, McAuliffe DJ, Kollias N, Flotte TJ, Doukas AG. Photomechanical delivery of 100?nm microspheres through the stratum corneum: Implications for transdermal drug delivery. Lasers Surg Med 2002;31:207-10.
- Goswami N, Joshi G, Sawant K. Floating microspheres of valacyclovir HCl: Formulation, optimization, characterization, in vitro and in vivo floatability studies. J Pharm Bioallied Sci 2012;4 Supp 1:S8-9.
- Almond BA, Hadba AR, Freeman ST, Cuevas BJ, Yorka AM, Detrisacb CJ, et al. Efficacy of mitoxantrone-loaded albumin microspheres for intratumoral chemotherapy of breast cancer. J Control Release 2003;91:147-55.
- Wang QS, Wang GF, Zhou J, Gao LN, Cui YL. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int J Pharm 2016;515:176-85.
- Yang TT, Cheng YZ, Qin M, Wang YH, Yu HL, Wang AL, et al. Thermosensitive chitosan hydrogels containing polymeric microspheres for vaginal drug delivery. Biomed Res Int 2017;2017:3564060.
- Didi A, Dadouch A, El Bekkouri H. Feasibility study for production of iodine-131 using dioxide of tellurium-130. Int J Pharm Pharm Sci 2016;8:e331.
- Taïeb D, Guille DA, Mundler LO. Guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. J Med Nucl 2008;32:101-10.
- Vitaux F. Thyroid gland irradiations and thyroid cancers critical bibliographic journal. J Med Nucl 2007;31:350-5.
- El Bez I. Cancer de la thyroïde et ablation par iode 131 sous thyrogen: Quand doser la thyroglobuline? Ann Endocrinol 2013;74:156.
- Spagnoli V, Azzalini L, Tadros VX, Picard F, Ly HQ. Contrast-induced nephropathy: An update. Ann Cardiol Angeiol 2016;65:87-94.
- Guerrouj H, Elamrani M, Ghfir I, Rais NB. Apport de l’iode 131 dans le traitement de l’adénome thyroïdien toxique. J Med Nucl 2012;36:561-4.
- Delmaire C. Imagerie des métastases cérébrales. Cancer Radiothér 2015;19:16-9.
- Shah B. Composites from agricultural detritus for pollution remedy. Int J Pharm Pharm Sci 2016;3:4-49.
- Boisserie G, Hasboun D. Utilisation de l’imagerie multimodalité en radiothérapie. Cancer Radiothé 2001;5:15-35.
- Belkacémi Y, Tsoutsou PG, Comet B, Kerrou K, Lartigau E. Évaluation de la radiosensibilité tumorale par l’imagerie fonctionnelle et métabolique: De la recherche à l’application clinique. Revue de la littérature. Cancer Radiothér 2006;10:124-33.
- Mbodj M, Guerrouj H, Amjad I, Rais NA. Contribution of radio-iodine 131 in the treatment of Grave’s Basedow disease in the department of nuclear medicine of Ibn sina hospital in Rabat. J Med Nucl 2009;33:592-8.
- Schlienger JL, Goichot B, Grunenberge F. Iode et fonction thyroïdienne. Rev Méd Int 1997;9:709-16.
- Hamed MA, Ghany AF, Osman NM. The diagnostic usefulness of FDG-PET/CT in detecting tumor recurrence not evident in whole body I-131 scan in differentiated thyroid carcinoma. Egypt J Radiol Nucl Med 2014;45:361-5.
- Eyles JE, Spiers ID, Williamson ED, Alpar HO, Williamson ED. Tissue distribution of radioactivity following intranasal administration of radioactive microspheres. J Pharm Pharmacol 2001;53:601-7.
- Nafea EH, El-Massik MA, El-Khordagui LK, Marei MK, Khalafallah NM. Alendronate PLGA microspheres with high loading efficiency for dental applications. J Microencapsul 2007;24:525-38.
- Kanafi MM, Ramesh A, Gupta PK, Bhonde RR. Dental pulp stem cells immobilized in alginate microspheres for applications in bone tissue engineering. Int Endod J 2014;47:687-97.
- Chang B, Ahuja N, Ma C, Liu X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater Sci Eng R Rep 2017;111:1-26.
- Keskar M, Sabatini C, Cheng C, Swihart MT. Synthesis and characterization of silver nanoparticle-loaded amorphous calcium phosphate microspheres for dental applications. Nano Adv 2019;1:627-35.
- Zhao XH, Tay FR, Fang YJ, Meng LY, Bian Z. Topical application of phenytoin or nifedipine-loaded PLGA microspheres promotes periodontal regeneration in vivo. Arch Oral Biol 2019;97:42-51.
- Park JH, Kim MK, Fiqi AE, Seo SJ, Lee EJ, Kim JH, et al. Bioactive and porous-structured nanocomposite microspheres effective for cell delivery: A feasibility study for bone tissue engineering. RSC Adv 2014;4:29062-71.


 Rahul Murlidhar mutadak *
Rahul Murlidhar mutadak *
 S. B. Gondkar
S. B. Gondkar



 10.5281/zenodo.12597829
10.5281/zenodo.12597829