Rilpivirine is non-nucleoside reverse transcriptase inhibitor (NNRTI) which is used for the treatment of HIV-1 infections in treatment-naive patients. It is a diarylpyrimidine derivative. The chemical name of rilpivirine is 4-{[4-({4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl}amino)-pyrimidinyl]amino}benzonitrilemonohydrochloride. Present review is focused on method conditions, linearity offered, sensitivity, accuracy, precision and assay results of various analytical methods such as UV-Visible spectroscopy, HPLC and LCMS for the estimation of rilpivirine.
Rilpivirine ,Linearity, Spectroscopic method, HPLC, LCMS.
Rilpivirine is non-nucleoside reverse transcriptase inhibitor (NNRTI) which is used for the treatment of HIV-1 infections in treatment-naive patients. It is a diarylpyrimidine derivative. The chemical name of rilpivirine is 4-{[4-({4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl}amino)-pyrimidinyl]amino}benzonitrilemonohydrochloride[3,4].
Its molecular is C22H18N6.
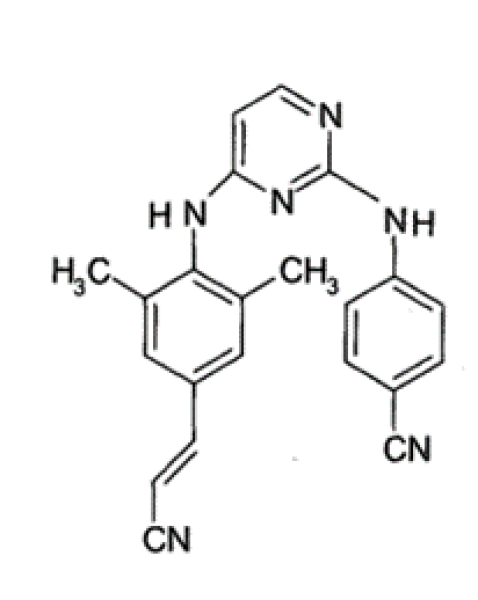
Figure 1: Chemical Structure of Ripivirine
Rilpivirine was developed by Tilbotec, Inc. and FDA approved on May 20, 2011. The internal conformational flexibility of rilpivirine and the plasticity of it interacting binding site gives it a very high potency and reduces the chance of resistance compared to other NNRTIS. Treatment of HIV-1 infections in treatment-naive patients with HIV-1 RNA ?100,000 copies/mL in combination with at least 2 other antiretroviral agents. Literature survey reveals few analytical methods for the estimation of rilpivirine such as chromatographic techniques [1-18], spectrophotometric techniques [19-21], hyphenated techniques [22-25]
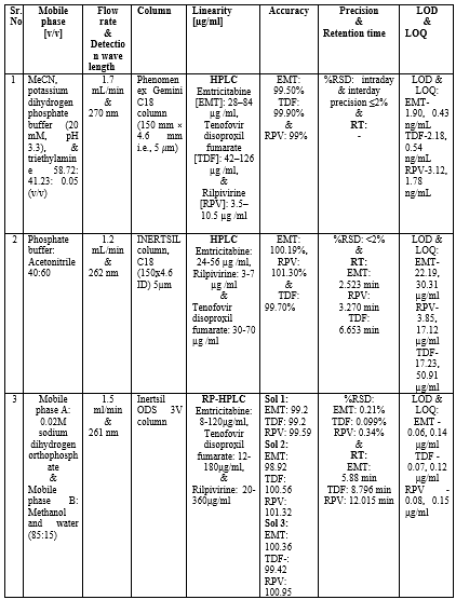
CHROMATOGRAPHIC TECHNIQUES1-18
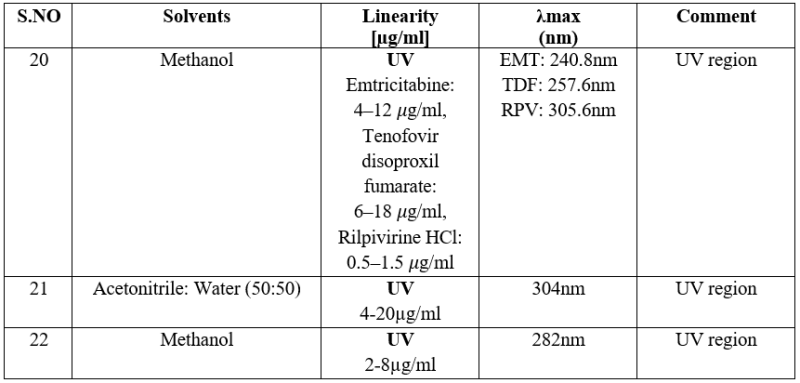
SPECTROPHOTOMETRIC TECHNIQUES19-21
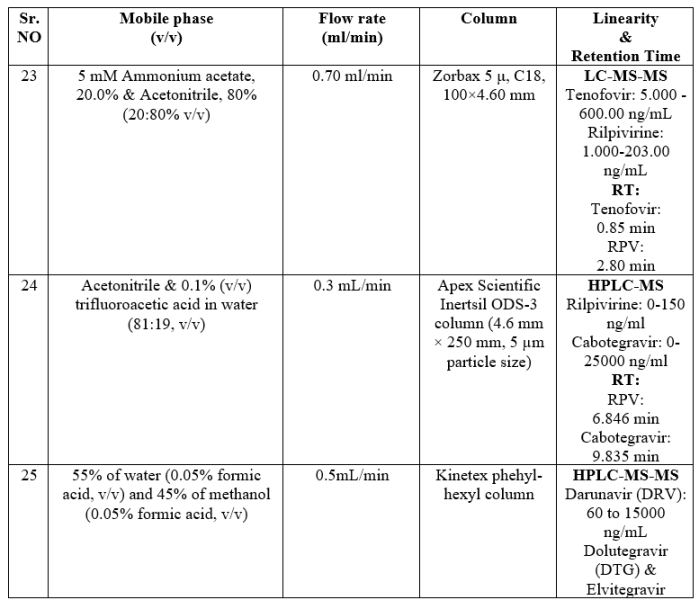
HYPHENATED TECHNIQUES22-25
CONCLUCSION:
Upon extensive literature survey, it was found that quite a good spectrophotometric, chromatographic as well as hyphenated techniques were reported for the quantification of Rilpivirine. Therefore, this study may help researchers in developing a simple, precise and robust method for the quantification of rilpivirine and its combinations.
REFERENCES
- Venkatesan S, Kannappan N, Mannemala SS. Stability-indicating HPLC method for the simultaneous determination of HIV tablet containing emtricitabine, tenofovir disoproxil fumarate, and rilpivirine hydrochloride in pharmaceutical dosage forms. Int Sch Res Notices. 2014.
- Karunakranth D, Midha AK, Babu RS, Kishore D. Development and validation of HPLC method for simultaneous estimation of emtricitabine, rilpivirine and tenofovir disoproxil fumarate tablet dosage form. IJRPB. 2018;6(1):8-15.
- Kavitha KY, Geetha G, Hariprasad R, Venkatnarayana R, Subramanian G. Development and validation of rp-hplc analytical method for simultaneous estimation of emtricitabine, rilpivirine, tenofovir disoproxil fumarate and its pharmaceutical dosage forms. IJCP. 2013;4(1):1.
- Kumar BR, Subrahmanyam KV. A validated stability-indicating rp-hplc method for the determination of rilpivirine. JGTPS. 2014;5(3):1822-6.
- Veeraswami B, Naveen VM. Development and validation of RP-HPLC method for the estimation of dolutegravir and rilpivirine in bulk and pharmaceutical dosage form and its application to rat plasma. Development. 2019;12(2).
- Kumar BM, Rajkamal B, Chandramowli B. Development and validation of Rilpivirine in pharmaceutical formulation by RP-HPLC. Am J PharmTech Res. 2019;9(03):345-53.
- Babu MN, Chandrasekar R. Development and validation of stability indicating RPHPLC method for the simultaneous estimation of dolutegravir and rilpivirine by forced degradation studies. Int. J. Pharm. Sci. & Res. 2021;12(9):4954-63.
- Pravalika P, Rani GT, Sree PT, Saritha Y. Development and validation of RP-HPLC method for the estimation of dolutegravir and rilpivirine in bulk and its tablet dosage form. J. Pharm. Res. 2021 jul;20(3):19.
- Ghosh S, Bomma S, Prasanna VL, Vidyadhar S, Banji D, Roy S. Method development and validation of rilpivirine in bulk and tablet doses form by RP-HPLC method. RJPT. 2013;6(3):240-3.
- Suneetha A, Lakshmi MV, Jyothi K, Priyanka BY. Development and validation of stability indicating RP-HPLC method for the simultaneous determination of cabotegravir and rilpivirine in bulk and injection dosage form. J. Pharm. Res. 2022 oct;21(4):145.
- Patel S, Nagappan K, Reddy GS. A new quantitative Reverse phase high-performance liquid chromatographic method for the quantification of Rilpivirine hydrochloride in bulk and dosage form. J. Appl. Pharm. Sci. 2018 Nov 30;8(11):157-62.
- Vemuluri PC, Dodda S. Stability indicating Reverse phase-high performance liquid chromatography method for simultaneous estimation of cabotegravir and rilpivirine. Ind. J. Pharm. Edu. Res. 2023;57(3s):s766-71.
- Prasanna A, Rani SS. A validated stability indicating RP-HPLC method development for simultaneous estimation of cabotegravir and rilpivirine in pharmaceutical dosage form: https://doi. Org/10.54037/wjps. 2022.101003. WJPPS. 2022 oct 1:22-9.
- Sudha T, Shanmugasundram P. Reverse phase high performance and HPTLC methods for the determination of rilpivirine bulk and in tablet dosage form. WJPPS. 2012 Jul 17;1(4):1183-96.
- Saminathan J, Vetrichelvan T. Development and validation of HPTLC method for simultaneous estimation of emtricitabine, rilpivirine and tenofovir disoproxil fumarate in combined dosage form. Bangladesh J. Pharmacol. 2016 Aug 10;19(1):114-21.
- Pasha SI, Varanasi MB, Mohammed I. Stability indicating RP-UPLC-PDA method development, validation of multi drug combination of emtricitabine, tenofovir alafenamide and rilpivirine in bulk drug and its tablet formulation. Orient. J. Chem. 2017 Jan 1;33(2):925-9.
- Khaleel N, Rahaman SA. Stability-indicating RP-UPLC method for simultaneous determination of dolutegravir and rilpivirine in bulk and pharmaceutical dosage form. Indian drugs. 2019 oct 1;56(10).
- Kumar KR, Suneetha A, Srilakshmi N. A validated LC method for the Estimation of Rilpivirine in API and Pharmaceutical Dosage form. J. Pharm. Res. 2012 Aug;5(8):4434-6.
- Sudha T, Shanmugasundram P. Reverse phase high performance and HPTLC methods for the determination of rilpivirine bulk and in tablet dosage form. World J. Pharm. Res. 2012 Jul 17;1(4):1183-96.
- Venkatesan S, Kannappan N. Simultaneous spectrophotometric method for determination of emtricitabine and tenofovir disoproxil fumarate in three-component tablet formulation containing rilpivirine hydrochloride. Int. Sch. Res Notices. 2014;2014.
- Ghurghure SM, Dhange A, Mhetre R, Phatak AA. Method development and validation of rilpivirine in bulk and solid dosage form by using uv-visible spectrophotometric method.
- Ghosh S, Kumar M, Jena S, Banji D, Roy S. Method development and validation of Rilpivirine in bulk and Pharmaceutical Tablet Dosage Form by using UV–Visible Spectrophometric Method. AJRC. 2012;5(12):1472-5.
- Kumar BM, Bigala BR. A Simple, selective, rapid and rugged method development and validation of tenofovir and rilpivirine in human plasma using liquid chromatography coupled with tandem mass spectrometry. EJPMR. 2017;4(7):605-13.
- Ramöller IK, Abbate MT, Vora LK, Hutton AR, Peng K, Volpe-Zanutto F, Tekko IA, Moffatt K, Paredes AJ, McCarthy HO, Donnelly RF. HPLC-MS method for simultaneous quantification of the antiretroviral agents rilpivirine and cabotegravir in rat plasma and tissues. J. Pharm. Biomed. Anal. 2022.10;213:114698.
- Zheng Y, Aboura R, Boujaafar S, Lui G, Hirt D, Bouazza N, Foissac F, Treluyer JM, Benaboud S, Gana I. HPLC-MS/MS method for the simultaneous quantification of dolutegravir, elvitegravir, rilpivirine, darunavir, ritonavir, raltegravir and raltegravir-?-d-glucuronide in human plasma. J. Pharm. Biomed. Anal. 2020.15;182:113119.


 Chaganti soujanya* 2
Chaganti soujanya* 2
 Srinija Naluka 1
Srinija Naluka 1




 10.5281/zenodo.10863534
10.5281/zenodo.10863534