This article offers a critical review of the evidence surrounding current therapeutic strategies for treating hyperuricemia. A variety of studies have been analyzed to assess the effectiveness of these treatments. Xanthine oxidase inhibitors, such as allopurinol and febuxostat, stand out as the most reliable and safe options for long-term management, making them the cornerstone of uric acid-lowering therapy. In contrast, the efficacy of uricosuric agents, which enhance renal excretion of uric acid, is significantly impacted by pharmacogenetic factors, meaning their effectiveness can vary between individuals based on genetic differences. Emerging therapies, including lesinurad and pegloticase, have demonstrated potential in the treatment of refractory hyperuricemia, particularly in acute cases. However, the current evidence supporting their use is limited to a few clinical trials, highlighting the need for more extensive, well-designed studies to establish clearer conclusions regarding their efficacy and safety in broader patient populations. These findings suggest a promising future for hyperuricemia treatment but emphasize the necessity for further research.
Crystal deposition disease, Hyperuricemia, Gout, Uric acid.
Hyperuricemia, the key factor in gout development, occurs when urate levels rise above saturation, leading to monosodium urate (MSU) crystal formation in joints. Uric acid, produced from purine metabolism, is balanced by daily excretion through feces and urine. Gout, a painful inflammatory arthritis, commonly affects the first metatarsophalangeal joint and results from excess urate deposition in tissues and joints. While hyperuricemia is the main cause of gout, only 5% of individuals with levels above 9 mg/dl develop the condition. Maintaining serum urate below 7 mg/dl is crucial for preventing gout and related conditions like diabetes, kidney damage, and cardiovascular disease velop luliconazole containing nanoemulgel and evaluate for topical drug delivery system.
PATHOPHYSIOLOGY
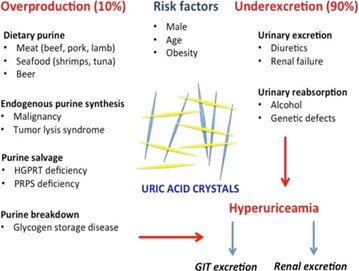
Uric acid, the final product of purine metabolism in humans, exists mostly as monosodium urate (MSU) due to the lack of uricase. The primary cause of hyperuricemia is underexcretion of MSU, accounting for 90% of cases, though overproduction of urate can also contribute. Elevated uric acid levels lead to MSU crystal formation, which can occur in asymptomatic patients. The exact trigger for acute gouty arthritis is unclear but likely involves the shedding of MSU crystals into the synovial fluid, leading to complement activation and inflammation. Acute gout attacks are influenced by the solubility of uric acid, which decreases in cold temperatures and lower pH. Estrogen offers protection by promoting uric acid clearance, while conditions like hypertension and diabetes predispose individuals to gout by impairing kidney function. Uric acid's poor solubility in acidic urine can also lead to stone formation.
GENES RESPONSIBLE FOR URIC ACID PRODUCTION
The SLC22A12 gene encodes the URAT1 transporter on renal tubule membranes, while SLC2A9 encodes another transporter regulating uric acid (UA) excretion. Polymorphisms in these genes reduce UA excretion, raising serum uric acid (SUA) levels. ABCG2 is a UA transporter in kidney tubular cells and the gastrointestinal tract. SLC17A1 and SLC17A3 are key genes influencing SUA levels by acting as membrane transporters in the kidneys. Other genes affecting SUA levels include SLC22A11, GCKR (glucokinase regulatory protein), LRRC16A (Carmil), and PDZK1.
PRODUCTION OF URIC ACID
Urate crystals form when uric acid levels in the blood become too high, leading to supersaturation. This involves several steps:
- Elevated Uric Acid Levels:
Hyperuricemia occurs when uric acid levels exceed the solubility threshold (around 6.8 mg/dL), leading to crystal formation due to overproduction or underexcretion.
- Supersaturation:
Uric acid becomes less soluble in cooler temperatures and lower pH, causing it to shift from a dissolved to a solid state when its solubility limit is surpassed.
- Nucleation:
Small clusters of uric acid molecules form, influenced by factors like pH and temperature, serving as the foundation for larger crystals.
- Crystal Growth:
Crystals grow as more uric acid molecules aggregate, depending on the concentration of uric acid and other substances in joint fluid.
DIAGNOSIS
Hyperuricemia, or elevated uric acid levels, is diagnosed through a blood test. A serum uric acid level of 8 mg/dL or higher indicates hyperuricemia, with normal levels usually below 6.8 mg/dL. A 24-hour urine collection may also be done to assess uric acid levels, and a purine-restricted diet test helps determine if high levels are due to diet, overproduction, or underexcretion. A spot urine sample can measure the uric acid-to-creatinine ratio, with a ratio above 0.8 indicating overproduction of uric acid. Hyperuricemia itself doesn't cause symptoms but can be linked to conditions like gout, nephrolithiasis, rheumatoid arthritis, hypothyroidism, and others.
Steps involved in diagnosis process
1 Clinical Evaluation:
A comprehensive medical history is taken, focusing on past joint pain episodes, family history of gout, and lifestyle factors such as diet and alcohol use.
The affected joints are examined for signs of inflammation, particularly the big toe, which is commonly affected during gout attacks.
2 Laboratory Tests:
Blood tests measure uric acid levels, with levels above 6.8 mg/dL indicating hyperuricemia. However, not all individuals with hyperuricemia develop gout.
In cases of joint inflammation, synovial fluid is aspirated and examined for needle-shaped monosodium urate crystals, which confirm gout.
- Complete Blood Count (CBC):
This test checks for signs of infection or other inflammatory conditions.
3 Imaging Studies:
Used to rule out other causes of joint pain and may show joint damage or tophi in chronic cases.
Detects urate crystals in joints and identifies tophi.
This specialized imaging technique visualizes urate crystal deposits in joints and surrounding tissues.
Causes of hyperuricemia ang gout
Hyperuricemia is the condition of having elevated levels of uric acid in the blood, which can lead to gout. Gout is a form of inflammatory arthritis that occurs when urate crystals accumulate in joints, leading to intense pain and swelling. Below are the common causes of hyperuricemia and gout.
Increased Production of Uric Acid:
Consuming purine-rich foods like red meat, organ meats, shellfish, and alcohol (especially beer) can lead to increased production of uric acid.
Some people have genetic mutations that make their bodies produce more uric acid or eliminate it less efficiently.
Conditions such as psoriasis, hemolytic anemia, and certain cancers can increase cell turnover, releasing more purines and increasing uric acid levels.
Decreased Elimination of Uric Acid:
The kidneys may not filter uric acid efficiently, leading to its accumulation. Chronic kidney disease or other renal disorders can impair the kidneys' ability to eliminate uric acid.
Drugs like diuretics (thiazides and loop diuretics), low-dose aspirin, and cyclosporine can reduce uric acid excretion.
When the body is dehydrated, less uric acid is excreted through the urine, leading to its buildup.
Underlying Medical Conditions:
A cluster of conditions like hypertension, insulin resistance, and hyperlipidemia is associated with higher uric acid levels.
High blood pressure is commonly associated with poor kidney function, leading to decreased uric acid elimination.
- Diabetes and Insulin Resistance:
These conditions can impair kidney function, reducing uric acid excretion.


 Dnyaneshwari Kure*
Dnyaneshwari Kure*
 Komal Chavan
Komal Chavan
 Kavita Kulkarni
Kavita Kulkarni
 Ashish Shalik Faltankar
Ashish Shalik Faltankar

 10.5281/zenodo.13958184
10.5281/zenodo.13958184