Abstract
Clarithromycin is a macrolide antibiotic it is used to treat a wide variety of bacterial infections this medication can also be used in combination with anti-ulcer medications to treat certain types of stomach ulcers. Clarithromycin often used in treating infection caused by Helicobacter pylori (H. pylori). These infections require the drug to remain in contact with the stomach lining for an extended period to be effective. GRDDS represent a cutting-edge advancement in pharmaceutical technology, designed to increase the time a drug spends in the stomach and upper GI tract. By prolonging gastric retention, these systems can enhance the absorption of drugs with a narrow absorption window, improve bio availability, reduce the frequency of dosing, and boost patient compliance. Floating tablets of clarithromycin where prepared by using a strategic blend of natural polymers, including xanthan gum, along with synthetic polymers like HPMC K4M and HPMC K100M. Sodium bicarbonate was included as a gas generating agent. The tablet was evaluated for tablet hardness, thickness, floating lag time, swelling index and T90%, were all with in the acceptable limits of USP specifications. The results demonstrated a significant impact of the polymer blend on T90%, effectively prolonging the availability of clarithromycin F6 in the stomach for up to 9.4 hours compared with other formulation and was concluded optimized one.
Keywords
Gastric residence time, Floating drug delivery system, Peptic ulcer disease
Introduction
Gastroretentive drug delivery systems (GRDDS) are a cutting-edge approach designed to boost the effectiveness of drugs absorbed mainly in the upper part of the gastrointestinal (GI) tract. These systems work by keeping the drug in the stomach longer, which enhances its absorption and overall effectiveness. By prolonging the time a drug stays in the stomach, GRDDS help ensure that medications, especially those that need to be absorbed in the stomach or the upper small intestine, have enough time to be fully absorbed before they move into areas of the GI tract where absorption is less efficient. This is particularly useful for drugs like clarithromycin, often used in treating infections caused by Helicobacter pylori (H. pylori). These infections require the drug to remain in contact with the stomach lining for an extended period to be effective [1]. H. pylori is a spiral-shaped, gram-negative bacterium that is a common culprit behind chronic gastritis, peptic ulcers, and even more severe conditions like gastric cancer and MALT lymphoma. Despite its widespread presence worldwide, treating this infection is becoming more challenging due to issues like antibiotic resistance, poor patient adherence to treatment, and inadequate drug delivery to the stomach lining where the bacteria reside. This rapid transit reduces the ability of the antibiotics to effectively target the bacteria embedded deep in the stomach’s mucus layer. Therefore, there is a growing need for new drug delivery systems that can improve gastric retention and provide sustained antibiotic release directly at the site of infection, enhancing treatment outcomes [2]. Peptic ulcer disease (PUD) is one of the most prevalent complications associated with infection, with up to 95% of duodenal ulcers and 70-90% of gastric ulcers linked to the bacterium. The standard eradication therapy aims to eliminate the organism, thereby reducing ulcer recurrence and promoting healing. However, conventional treatment regimens frequently fall short, achieving less than the desired 80% eradication rates set by international guidelines. This suboptimal efficacy is often attributed to the inability of traditional dosage forms to maintain adequate drug levels in the stomach for a sufficient period, necessitating the exploration of alternative delivery strategies.
Significance of Gastroretentive Drug Delivery Systems:To tackle the issues of poor drug retention and low treatment success rates in the stomach, gastroretentive drug delivery systems (GRDDS) have emerged as a promising strategy to enhance the effectiveness of eradication therapies. These innovative systems are specifically designed to increase the time that drugs remain in the stomach, enabling a sustained and localized release of antibiotics. GRDDS come in various forms, including floating tablets, bioadhesive formulations, expandable devices, and high-density systems, each utilizing different mechanisms to extend gastric residence time.
Gastroretentive Drug Delivery Systems (GRDDS)
GRDDS represent a cutting-edge advancement in pharmaceutical technology, designed to increase the time a drug spends in the stomach and upper GI tract. By prolonging gastric retention, these systems can enhance the absorption of drugs with a narrow absorption window, improve bioavailability, reduce the frequency of dosing, and boost patient compliance. The principle behind GRDDS is to overcome the limitations of conventional oral drug delivery systems, which often face rapid gastric emptying. This rapid transit can lead to the premature release of drugs into the intestines, where absorption might be less efficient or entirely inadequate. [3] GRDDS address this by ensuring that the drug remains in the stomach for a controlled duration, allowing it to be released steadily and absorbed effectively at the intended site. Table-1, will compare the various mechanisms used in GRDDS, highlighting their advantages and challenges. [4,5]
Challenges in Developing Gastroretentive Drug Delivery Systems
While GRDDS offer significant benefits, their development presents several challenges: [5,7]
- Formulation Challenges: Developing a formulation that remains in the stomach for an extended period without disintegrating or losing its buoyancy is challenging. The selection of polymers, excipients, and preparation methods must be carefully optimized to achieve the desired retention and release profile.
- Patient Variability: The effectiveness of GRDDS can vary depending on individual patient factors such as gastric motility, pH, and the presence of food, which can affect floating behavior, retention time, and drug release rates.
- Regulatory Challenges: Developing GRDDS requires extensive in vitro and in vivo testing to demonstrate safety, efficacy, and stability, which can be time-consuming and costly.
- Gastric Emptying: The unpredictability of gastric emptying, particularly in patients with certain medical conditions, can impact the retention time of GRDDS, leading to premature drug release into the intestines.
MATERIALS AND METHODS
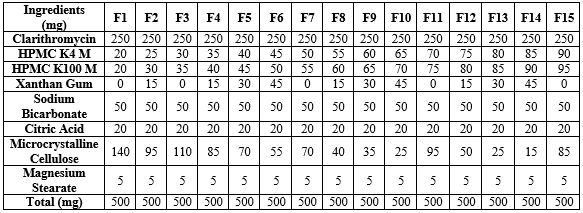
Clarithromycin - purchased from microlabs limited,bangalore,HPMC K100M- purchased from Otto chemie pvt Ltd, Mumbai,HPMC K4M- purchased from Otto chemie pvt Ltd , Mumbai, Xanthan gum- purchased from SD Fine chemical Ltd, Mumbai, Sodium bicarbonate(NaHCO3)- purchased from SD Fine chemicals Ltd,Mumbai , Citric acid- purchased from SD Fine chem Ltd Mumbai, Microcrystalline cellulose (MCC)- purchased from Loba chemical pvt Ltd, Mumbai, Magnesium stearate(MS)- purchased from Loba chemie pvt Ltd, Mumbai.
FORMULATION
Drug-Excipients Compatibility, External Appearance, and Size Uniformity Studies
Drug-Excipients Compatibility Study:
The compatibility of Clarithromycin with selected excipients (HPMC K4M, HPMC K100M, Xanthan gum) will be evaluated using FTIR and DSC analyses. FTIR spectra will be recorded for Clarithromycin, individual excipients, and their physical mixtures (drug: excipient ratio of 1:1) in the range of 500-4000 cm??1; using a Shimadzu 8400S spectrophotometer. Characteristic peaks will be identified and compared to assess any drug-excipient interactions. DSC thermograms will be recorded using a DSC 204 F1 Phoenix® under a nitrogen atmosphere, heating the samples at 10°C/min. The retention of Clarithromycin's melting point in the physical mixtures will indicate compatibility.
External Appearance and Size Uniformity Studies:
The external appearance of the floating tablets will be visually inspected for color, shape, and any defects. The uniformity in size will be assessed by measuring the diameter of 20 randomly selected tablets using a digital Vernier caliper. The results will be expressed as the mean diameter ± standard deviation. [8.9]
Precompression Parameters of Clarithromycin Floating Tablets Formulation Powder Blend
Angle of Repose [?]:
The angle of repose will be determined by allowing the powder blend to flow freely through a funnel onto a flat surface. [10] The height (h) and radius (r) of the resulting powder cone will be measured, and the angle of repose will be calculated using the formula:
? = tan?1(h/r)
An angle of repose less than 30° indicates good flow properties, while an angle greater than 40° suggests poor flow.
Loose Bulk Density (LBD) [gm/cm?3;]:
The loose bulk density will be determined by gently pouring the powder blend into a graduated cylinder and measuring its volume. The weight of the powder will be recorded, and LBD will be calculated using the formula:
LBD = Weight of the powder / Volume of the powder
Tapped Bulk Density (TBD) [gm/cm?3;]: The tapped bulk density will be measured by tapping the cylinder containing the powder blend 100 times or until no further volume change occurs. The weight and final volume will be recorded, and TBD will be calculated using the formula:
TBD = Weight of the powder / Tapped volume of the powder
Hausner’s Ratio (HR):
Hausner’s ratio will be calculated as the ratio of TBD to LBD:
HR = LBD / TBD
A Hausner’s ratio less than 1.25 indicates good flowability, whereas a ratio greater than 1.5 indicates poor flowability.
Compressibility Index [%]:
The compressibility index will be calculated using Carr’s index formula:
Compressibility Index = [(TBD - LBD) / TBD] × 100
A compressibility index below 15% indicates good flow properties, while values above 25% suggest poor flow.
Post-Compression Characteristics of Clarithromycin Floating Tablet Formulations
Thickness (mm):
The thickness of 20 randomly selected tablets will be measured using a Vernier caliper. The mean thickness and standard deviation will be recorded.
Hardness (kg/cm?2;): Tablet hardness will be determined using a Monsanto hardness tester. Ten tablets will be tested, and the average hardness will be reported.
Friability (%):
Friability will be assessed using a Roche friabilator. Twenty pre-weighed tablets will be rotated at 25 rpm for 4 minutes. [11] The tablets will be reweighed, and the friability will be calculated as:
Friability (%) = [(Initial weight - Final weight) / Initial weight] × 100
A friability of less than 1% is generally considered acceptable.
Weight Variation (mg):
Twenty tablets will be randomly selected and individually weighed. The average weight and percentage deviation from the mean will be calculated. According to pharmacopeial standards, the tablets must meet specified limits for weight variation.
In-Vitro Drug Release:
The in-vitro drug release study will be conducted using a USP Type II dissolution apparatus (Electrolab, TDT-06T, India). Tablets will be placed in 900 mL of 0.1N HCl maintained at 37.0 ± 0.5°C and stirred at 50 rpm. Aliquots (5 mL) will be withdrawn at specified time intervals, filtered, and analyzed for Clarithromycin content using a UV-Vis spectrophotometer at 280 nm. Fresh dissolution medium will be added to maintain the volume. The cumulative percentage of drug release will be plotted against time, and the release kinetics will be analyzed. [12-16]
Accelerated Stability Testing According to ICH Q1A(R2)
Stability Testing Procedure: The optimized formulation will undergo accelerated stability testing as per ICH Q1A(R2) guidelines. [62-66] The tablets will be packed in HDPE bottles and stored at 40°C ± 2°C/75% RH ± 5% RH for six months. Samples will be withdrawn at 0, 3, and 6 months and evaluated for physical appearance, drug content, FLT, and in-vitro drug release. The data will be analyzed to determine the stability of the formulation over time.
Comparison with Marketed Drug
Comparison with Marketed Clarithromycin Tablets: The drug release profile of the optimized formulation will be compared with that of a marketed Clarithromycin tablet. [17-19] Both formulations will be subjected to in-vitro dissolution testing under identical conditions. The similarity factor (f2) will be calculated to compare the dissolution profiles, with values between 50 and 100 indicating similarity between the formulations.
Floating Lag Time and Swelling Index
Floating Lag Time (FLT):
The FLT will be determined by placing the tablet in 200 mL of 0.1N HCl at 37.0 ± 0.5°C and observing the time taken for the tablet to rise to the surface and float. The duration will be recorded in seconds.
Swelling Index:
The swelling index will be determined by placing a tablet in a beaker containing 200 mL of 0.1N HCl at 37.0 ± 0.5°C. At predetermined intervals, the tablet will be removed, blotted to remove excess fluid, and reweighed. The swelling index (SI) will be calculated using the formula:
SI = [(Final weight - Initial weight) / Initial weight] × 100
The results will be plotted against time to assess the swelling behaviour over the duration of the test
RESULTS AND DISCUSSION
PRE FORMULATION STUDIES
Fourier Transform Infrared (FTIR) Spectroscopy
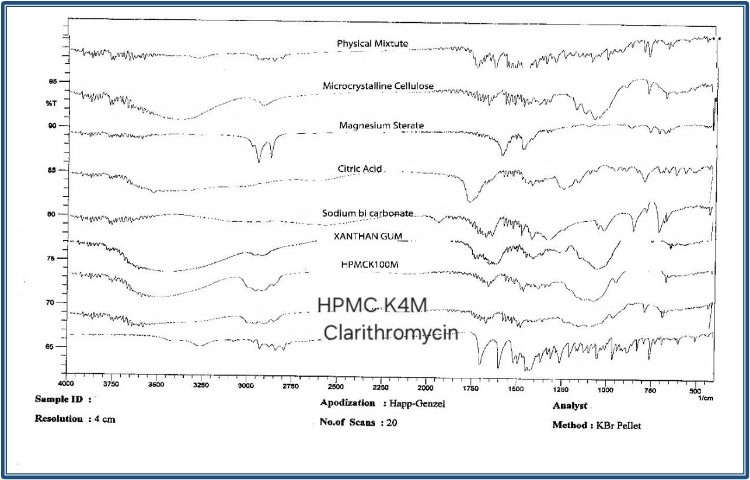
Fig-1: FTIR spectra of clarithromycin, excipients and physical mixtures.
FTIR analysis was performed to assess the compatibility between Clarithromycin and the selected excipients. FTIR spectra of Clarithromycin, individual excipients, and physical mixtures (Clarithromycin: Excipients ratio of 1:1) were recorded using an FTIR. (Fig-3) The samples were scanned over a wavelength range of 500-4000 cm??1;. The characteristic peaks of Clarithromycin were identified and compared with the physical mixtures to detect any potential interactions between the drug and excipients. The FTIR spectra of the physical mixtures retained the key peaks of Clarithromycin without significant shifts, indicating no major drug-excipient interactions.
Differential Scanning Calorimetry (DSC)
DSC was used to further assess the compatibility of Clarithromycin with the excipients. DSC thermograms of Clarithromycin and its physical mixtures (Clarithromycin: Excipients ratio of 1:1) were obtained using a DSC. The samples were heated at a rate of 10°C/min under a nitrogen atmosphere.
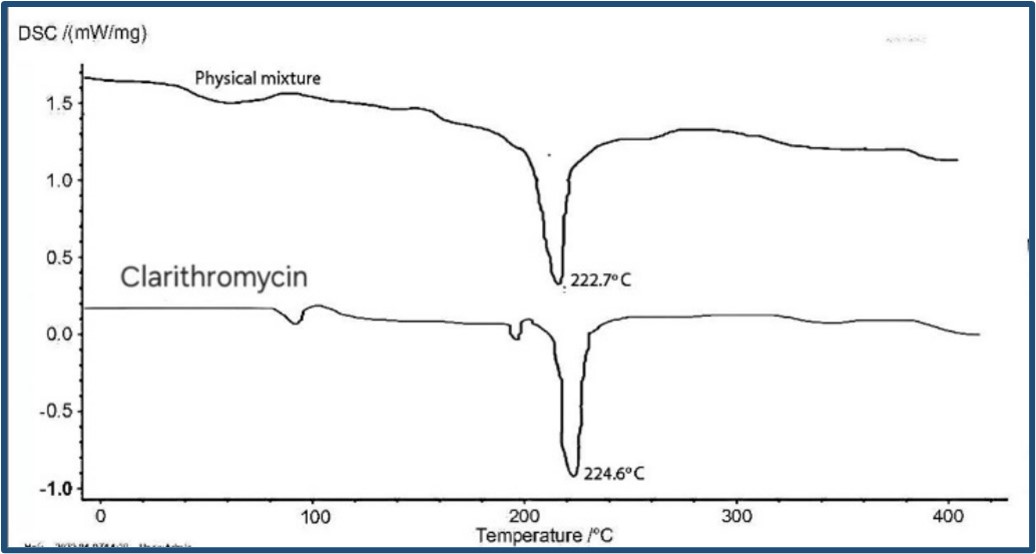
Fig-2: DSC thermograms of clarithromycin and physical mixture.
Clarithromycin exhibited a sharp endothermic peak corresponding to its melting point at 224.6°C. The DSC thermograms of the physical mixtures showed the presence of the Clarithromycin melting point peak without any significant shifts or additional peaks, indicating the absence of interactions between clarithromycin and the excipients.
UV-Visible Spectrophotometry
The absorbance of Clarithromycin was measured using a UV-visible spectrophotometer. Clarithromycin solutions were prepared in suitable solvents and scanned in the wavelength range of 200-400 nm. The maximum absorbance was observed at 210 nm.
Table-3: Standardisation of clarithromycin.
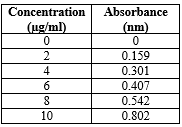
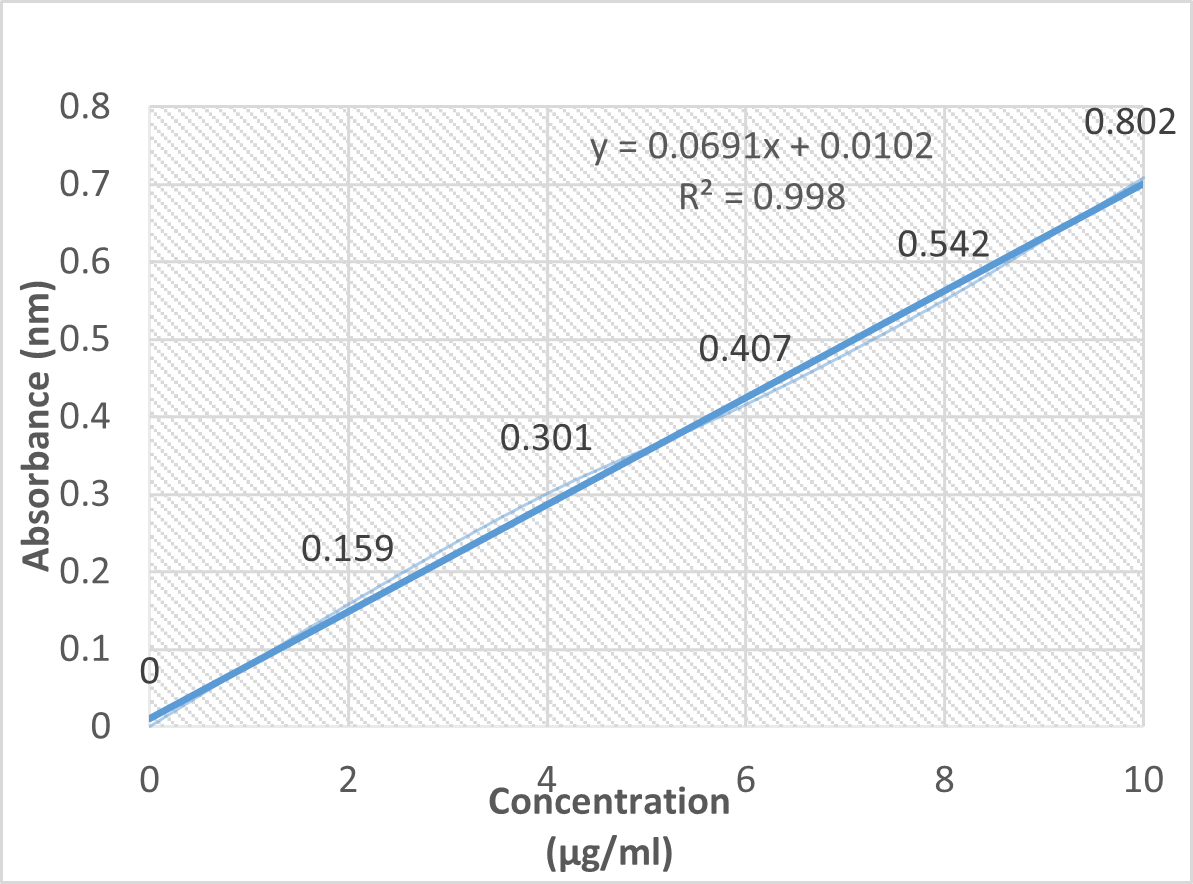
Fig-3: Standard calibration curve of clarithromycin.
A standard calibration curve of Clarithromycin was plotted over the concentration range of 0 to 10 ?g/mL.
- The absorbance data demonstrated linearity with an R?2; value of 0.998, indicating compliance with Beer’s Law.
Formulation Development of Clarithromycin Floating Tablets
The formulation development of Clarithromycin floating tablets involved the use of key excipients to achieve optimal floating and controlled drug release characteristics. The formulation included HPMC K4, HPMC K100, Xanthan gum, Sodium Bicarbonate, Citric Acid, Microcrystalline Cellulose, and Magnesium Stearate. These ingredients were carefully selected to enhance the performance of the floating tablets. HPMC K4, HPMC K100, and Xanthan gum served as matrix-forming agents that controlled the drug release rate. Sodium Bicarbonate and Citric Acid were included as gas-generating agents to ensure the buoyancy of the tablets. Microcrystalline Cellulose acted as a filler, providing structural integrity, while Magnesium Stearate was used as a lubricant to facilitate the tablet manufacturing process. The development aimed to optimize key formulation parameters such as Swelling Index, Floating Lag Time, and the time required to release 90% of the drug, ensuring an effective and reliable gastroretentive drug delivery system.
Preparation of Clarithromycin Floating Tablets
Clarithromycin was mixed with the selected quantities of HPMC K4M, HPMC K100M, Xanthan gum, Sodium bicarbonate, Citric acid, Microcrystalline cellulose, and Magnesium stearate. (Table-3) The mixture was triturated for 5 minutes in a mortar to ensure uniform distribution of the drug and excipients.
The blend was then subjected to direct compression using a 10-station rotary tablet compression machine (PROTON MINI PRESS) equipped with a 9 mm standard flat-
Table-1: Preparation of Clarithromycin Floating Tablets
Precompression Properties
Angle of Repose:
The angle of repose values for the formulations range between 25.63° and 30.82°, indicating good to acceptable flowability of the powder blends. Formulations with lower angles (F12) exhibit better flow properties, which are desirable for ensuring consistent die filling during tablet compression. Higher angles (e.g., F4 and F14) suggest slightly poorer flow, which could affect the uniformity of the tablets if not properly controlled.
Loose and Tapped Bulk Densities:
The loose bulk density values range from 0.95 to 1.10 gm/cm?3;, while the tapped bulk density ranges from 1.17 to 1.40 gm/cm?3;. The relatively close values of loose and tapped densities indicate that the powder blends have good packing properties, which is beneficial for consistent tablet formation. Formulations with higher tapped density (e.g., F4 and F14) might require adjustments in compression force to avoid over-compaction.
Hausner’s Ratio:
The Hausner’s ratio values mostly lie between 1.11 and 1.33, reflecting moderate flowability. Ratios closer to 1, such as in F3 and F9, indicate excellent flow properties, which are critical for uniform powder flow during tablet formation. Higher ratios in formulations like F4 and F5 suggest the need for flow enhancers or optimization of the blending process.
Compressibility Index:
The compressibility index ranges from 10.52% to 25%, indicating good compressibility overall. Formulations with lower compressibility indices (e.g., F3 and F9) exhibit better compaction behavior, essential for forming tablets with adequate mechanical strength. Higher values, such as in F4 and F5, might indicate more compressible but less flowable powder, which may require adjustments during the compression process.
Table-2: Precompression Properties
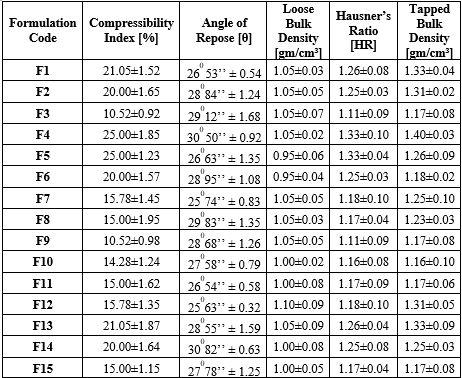
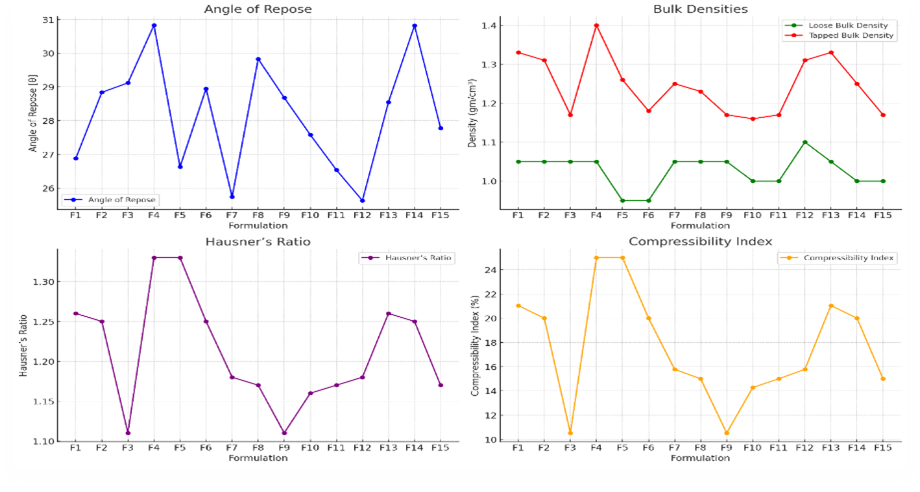
Fig-4: Precompression properties analysis
Post compression
Thickness:
Tablet thickness across formulations ranges between 3.10 mm and 3.29 mm, showing consistent manufacturing control. Consistent thickness is crucial for uniformity in appearance and mechanical properties. Variations within acceptable limits suggest stable compression parameters across batches.
Hardness:
The hardness values range from 4.44 to 4.71 kg/cm?2;, reflecting adequate mechanical strength suitable for handling, packaging, and transport. This range ensures that the tablets are not too hard to impede dissolution nor too soft to break easily, providing a balanced strength profile.
Table-3: Post compression Properties
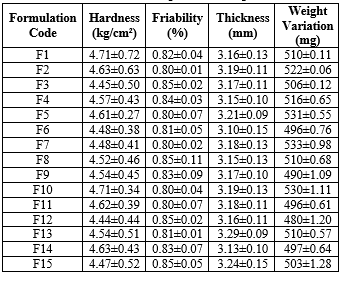
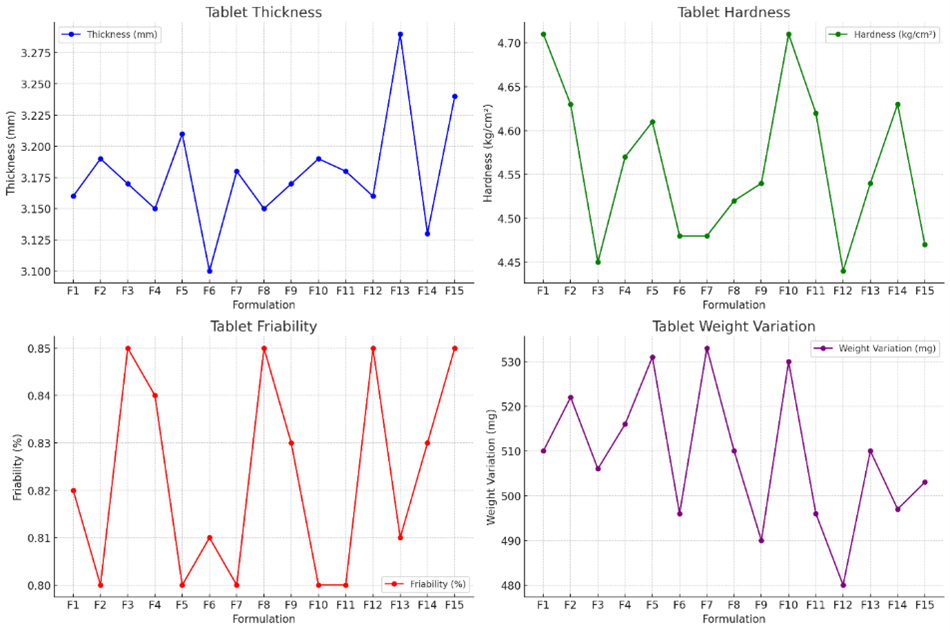
Fig-5: Post compression properties
Friability:
Friability values are all below 1%, indicating that the tablets have good resistance to chipping and breaking under mechanical stress. Lower friability is particularly important for gastroretentive tablets, which must maintain integrity for extended periods in the stomach.
Weight Variation:
Weight variation among the tablets is within acceptable pharmaceutical limits, ranging from 480 mg to 533 mg. This uniformity ensures dose consistency, which is crucial for therapeutic efficacy, especially in controlled release formulations.
Evaluation of Floating Clarithromycin Tablet
Table 4: Formulation and evaluation of floating clarithromycin tablet
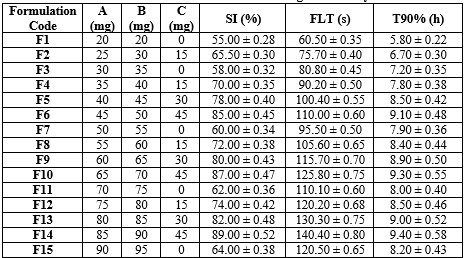
Floating Lag Time (FLT):
FLT values generally increase with higher amounts of polymers, particularly when Xanthan Gum is included, due to its high swelling and viscosity which enhance buoyancy.
Swelling Index (SI):
SI values increase with the presence of Xanthan Gum, as it significantly contributes to the swelling properties of the tablets. Formulations without Xanthan Gum show relatively lower SI values.
T90% (h):
T90% values reflect the extended release due to the combined effect of HPMC K4 M, HPMC K100 M, and Xanthan Gum. The higher the amount of these polymers, the longer it takes to release 90% of the drug.
The formulation was optimized to achieve the desired floating duration, controlled drug release, and tablet stability. To optimize the formulation of gastroretentive floating tablets, the analysis focused on key criteria including minimizing floating lag time (FLT), maximizing the swelling index (SI), prolonging the time to 90% drug release (T90%), and achieving a high R?2; value for Zero Order kinetics, which indicates controlled and consistent drug release. A multi-criteria decision analysis was used, weighing the importance of each factor based on the desired goals, such as prioritizing FLT and T90% for optimal performance in gastroretentive systems. The optimization identified Formulation F6 as the optimal choice, exhibiting a balanced combination of a reasonable FLT of 82.41 seconds, a high SI of 88.00%, a prolonged T90% of 9.10 hours, and a high Zero Order R?2; of 0.9980, which reflects excellent controlled release behavior. These attributes make Formulation F6 the best candidate for an optimized gastroretentive floating tablet, offering a well-rounded performance profile with consistent, extended drug release and robust matrix formation.
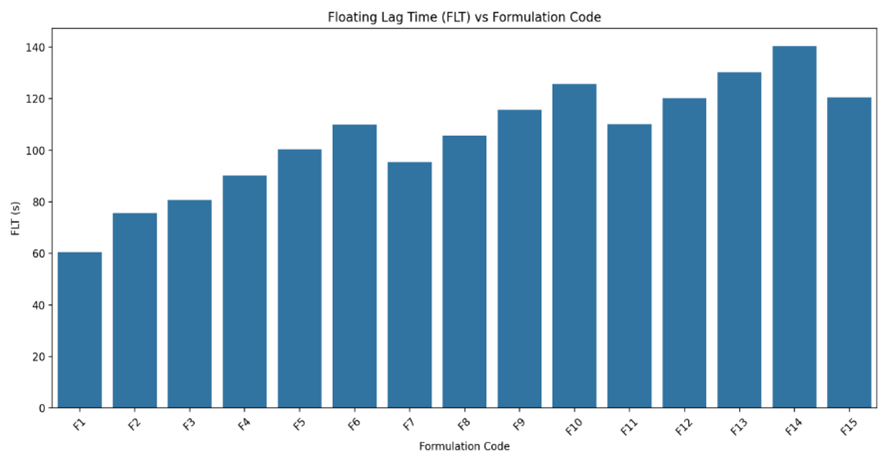
Fig-5: Floating Lag Time of Clarithromycin Floating Tablets (F1 to F15) using HPMC K4M, HPMC K100 and Xanthan gum.
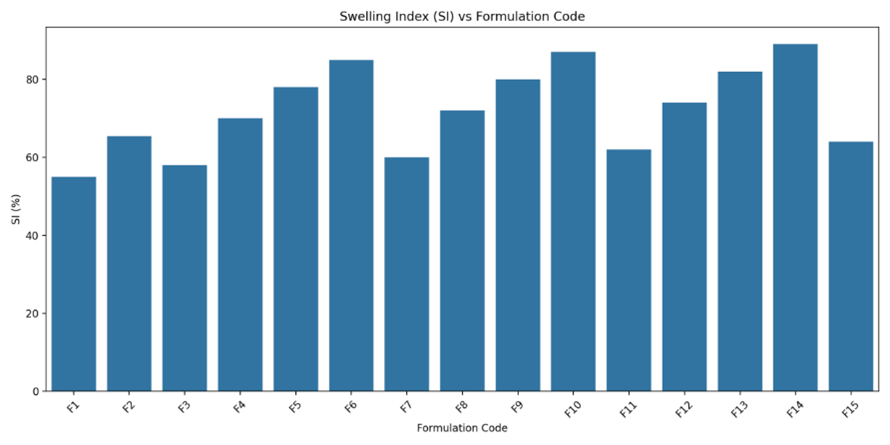
Fig-6: Swelling index of Clarithromycin Floating tablets (F1 to F15) using HPMC K4M, HPMC K100M and Xanthan gum.
In Vitro drug release
Fig-5, is the graph showing the T90% (h) for formulations F1 to F5. The plot indicates the mean T90% values along with their standard errors. The trend suggests a gradual increase in T90% from formulation F1 to F5, indicating longer time durations for 90% of the formulation release as the formulation number increases.
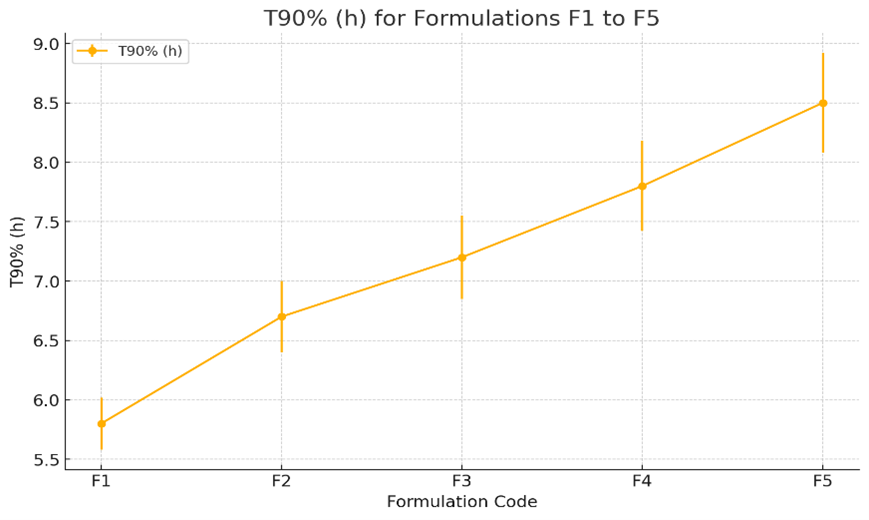
Fig-7: In-vitro drug release profile of Clarithromycin floating tablets formulations(F1-F5) - HPMC K4M, HPMC K100M and Xanthan gum
Fig-6, is the graph showing the T90% (h) for formulations F6 to F10. The plot illustrates the mean T90% values along with their standard errors, showing variations across different formulations. The trend indicates some fluctuations in F7-F9 the T90% values, with formulation F10 showing the highest value in this formulation.
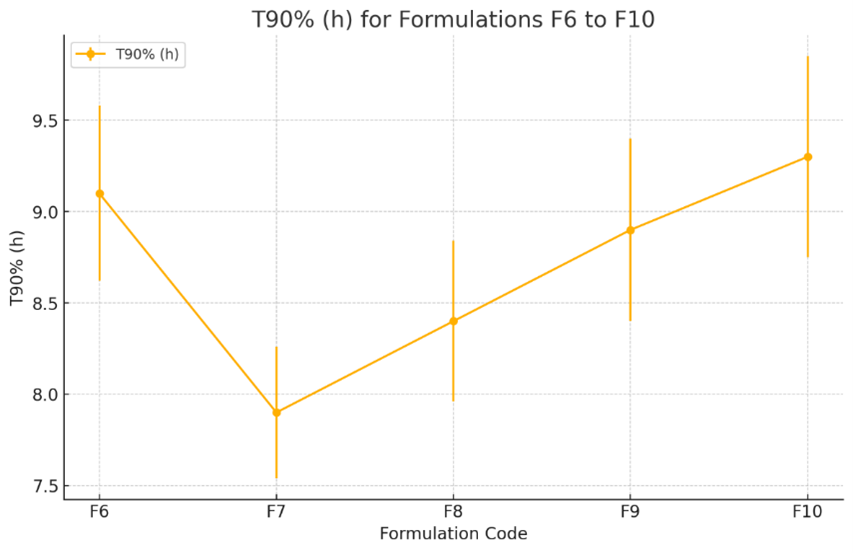
Fig-8: In-vitro drug release profile of Clarithromycin floating tablets formulations(F1-F5) - HPMC K4M, HPMC K100M and Xanthan gum
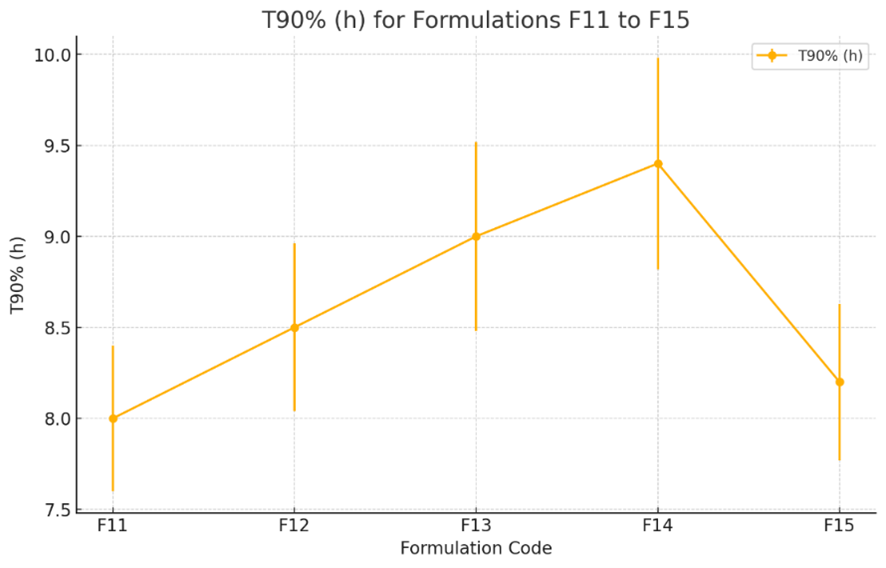
Fig-9: In-vitro drug release profile of Clarithromycin floating tablets formulations(F1-F5) - HPMC K4M, HPMC K100M and Xanthan gum
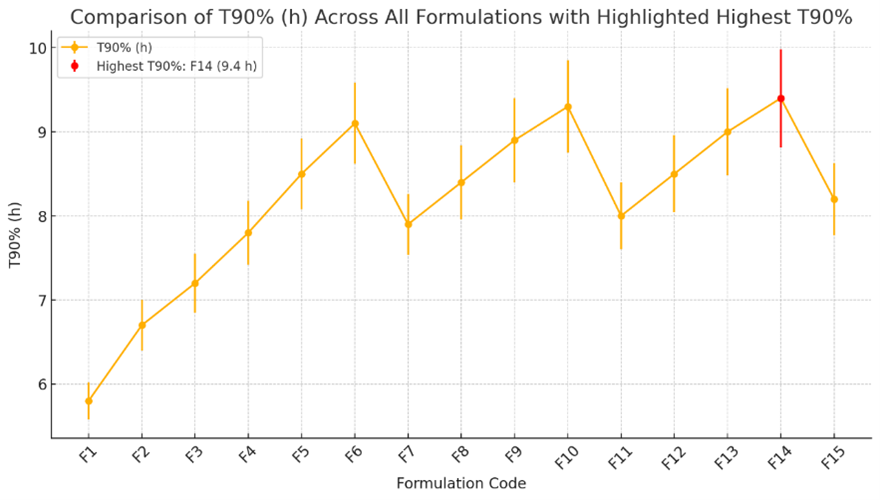
Fig-10: In-vitro drug release profile Comparison of Clarithromycin floating tablets formulations (F1-F15) - HPMC K4M, HPMC K100M and Xanthan gum
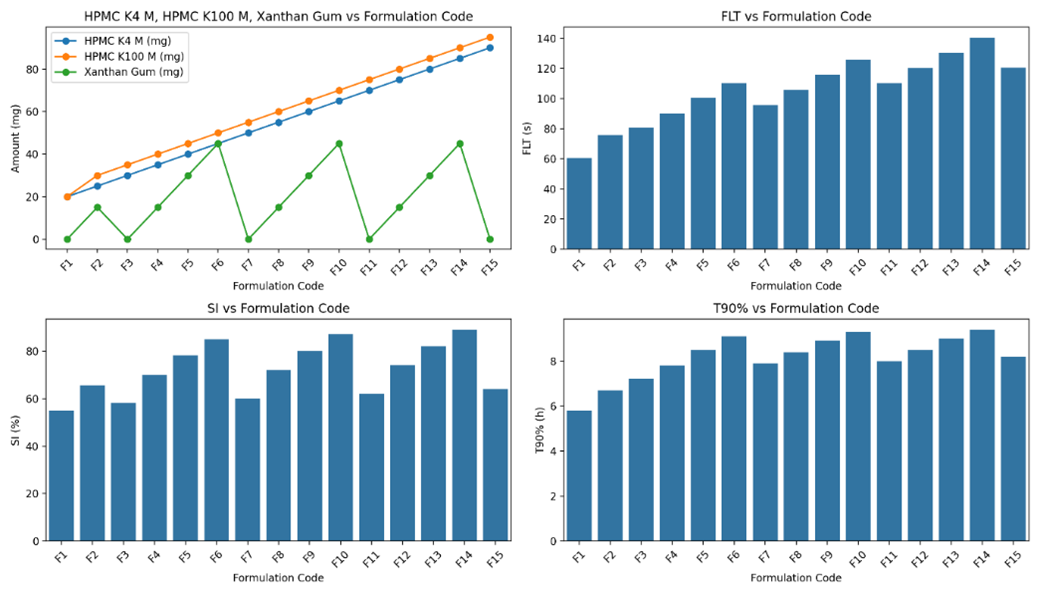
Fig-11: Comparing the formulation and evaluation of Clarithromycin floating tablets formulations(F1-F15) - HPMC K4M, HPMC K100M and Xanthan gum
Table 5: Kinetics of clarithromycin floating tablets
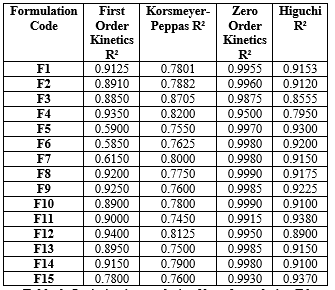
Table 6: Optimization analysis of best formulation F6
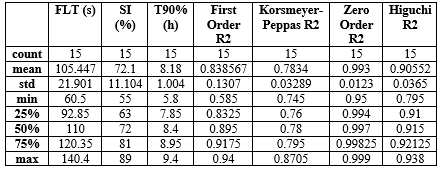
The optimization analysis identified Formulation F6 as the optimal choice based on the weighted criteria: FLT (s): 82.41 seconds, SI (%): 88.00% and T90% (h): 9.10 hours
Zero Order R?2;: 0.9980 (high fit indicating consistent, controlled release)
Formulation F6 offers a balanced combination of a reasonable floating lag time, high swelling capacity, extended release duration, and a strong alignment with Zero Order kinetics, making it the best candidate for an optimized gastroretentive floating tablet.
First Order Kinetics (R?2;):
Generally lower R?2; values in formulations with higher HPMC content due to the impact of sustained release mechanisms overriding the initial burst release patterns.
Korsmeyer-Peppas (R?2;):
Reflects diffusion-controlled release, with higher values seen in formulations with higher polymer ratios, indicating a more complex release profile due to swelling and matrix erosion.
Zero Order Kinetics (R?2;):
Shows high values across all formulations, indicating a constant drug release rate, particularly for those with higher Xanthan Gum content that enhances sustained release behavior.
Higuchi (R?2;):
Consistently high values suggest a diffusion-controlled release, particularly in formulations with higher HPMC and Xanthan Gum ratios that provide a thickening effect, enhancing the sustained release profile.
Accelerated Stability Testing (ICH Q1A(R2))
The Developed formulation F6 were stored in a stability chamber (Remi CHM-10S, INDIA) at 40±20C and humidity of 75% RH for 6 months and observed for the Floating Lag Time (FLT), Swelling index (SI), Time required to release 90% of the drug (T90 %) and in-vitro drug release 0, 30, 90, 180 days. F6 formulation showed better SI, T90% than the all F1-F15 batches, Hence, F6 formulation selected for Further studies. The zero times samples were used as controls.
Table 7: Accelerated Stability Testing (ICH Q1A(R2)
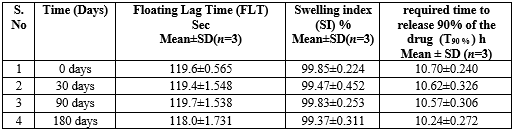
The developed formulation of clarithromycin floating tablets F6 remains relatively stable across all time points, with slight fluctuations observed after storage. SI also shows no changes, maintaining a high swelling index throughout the study period. T90% shows a slight decreasing trend over time, indicating a marginal reduction in the time required to release 90% of the drug after extended storage. These results suggest that F6 maintains consistent performance in terms of FLT and SI, with only a minor impact on T90?ter prolonged storage. Fig-7. Shows the comparison of Floating Lag Time (FLT), Swelling Index (SI), and Time required to release 90% of the drug (T90%) for the F6 formulation over different time points (0, 30, 90, and 180 days).
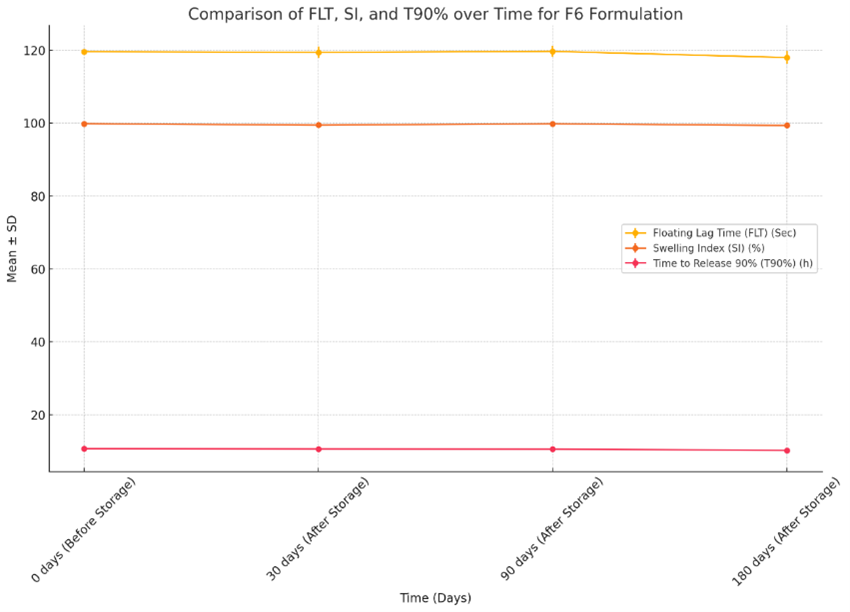
Fig-12. Comparison of Floating Lag Time (FLT), Swelling Index (SI), and Time required to release 90% of the drug (T90%) for the F6 formulation.
Comparison of Clarithromycin floating tablet vs Marketed tablet
The Developed formulation of Clarithromycin floating tablets F6 was compared with the marketed conventional tablet (Claribid 250, Abbott) of Clarithromycin 250mg. Fig-5 graph compares the time required to release 90% of the drug (T90%) between the developed Clarithromycin F6 floating tablets and the marketed conventional Clarithromycin 250 mg tablets. The F6 floating tablets have a significantly longer T90% (10.24 hours) compared to the conventional tablets, which release 90% of the drug in just 2.50 hours. This highlights the sustained release advantage of the F6 floating formulation, which can provide prolonged therapeutic effects.
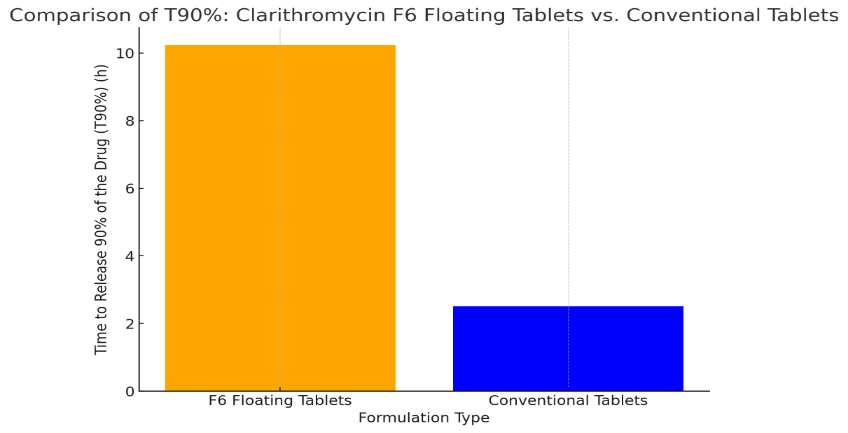
Fig-13. Comparison of Clarithromycin floating tablet vs Marketed tablet.
CONCLUSION
In this study, a statistically optimized gastroretentive floating tablet of clarithromycin was developed and evaluated for its in vitro performance. The floating tablets were formulated using a strategic blend of natural polymers, including Xanthan gum, along with synthetic polymers like HPMC K4M and HPMC K100M. The results demonstrated a significant impact of the polymer blend on T90%, effectively prolonging the availability of clarithromycin F6 in the stomach for up to 9.4 hours. Key evaluation parameters, such as tablet hardness, thickness, floating lag time, swelling index, and T90%, were all within the acceptable limits of USP specifications. The extended gastric residence time of clarithromycin in the optimized formulation suggests that the drug could maintain consistent therapeutic levels in the stomach, thereby enhancing its bioavailability and maximizing the utilization of the administered dose. Thus, the optimized clarithromycin floating tablet formulation F6 shows promise in providing sustained drug release, ensuring prolonged gastric retention, and potentially improving the clinical efficacy of clarithromycin in the treatment of gastric infections.
REFERANCE
- Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208(2):233-248.
- Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 1995;9(2):59-69
- .Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212-239.
- Malfertheiner P, Megraud F, Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6-30.
- Navjot K, Rakesh K, Amita S, Sinha VR. Preparation and evaluation of floating tablets of pregabalin. Drug Dev Ind Pharm. 2016;42(4):654-660.Jun 10;168(2):151-65.
- Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. International journal of pharmaceutics. 2016 Aug 20;510(1):144-58.
- Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. International journal of pharmaceutics. 2016 Aug 20;510(1):144-58
- Narendra C, Srinath MS, Babu G. Optimization of bilayer floating tablet containing metoprolol tartrate as a model drug for gastric retention. AApS pharmSciTech. 2006 Jun;7:E23-9.
- Myers RH, Montgomery DC, Anderson-Cook CM. Response surface methodology: process and product optimization using designed experiments. John Wiley & Sons; 2016 Jan 4.
- Riswanto FD, Rohman A, Pramono S, Martono S. Application of response surface methodology as mathematical and statistical tools in natural product research. Journal of Applied Pharmaceutical Science. 2019 Oct 6;9(10):125-33.
- Das U, Panda DK, Mandal S. Formulation by design: An overview. Drug formulation design. 2023 Jan 24.Diwekar UM. Introduction to applied optimization. Springer Nature; 2020 Oct 29.
- Gabrielsson J, Lindberg NO, Lundstedt T. Multivariate methods in pharmaceutical applications. Journal of Chemometrics: A Journal of the Chemometrics Society. 2002 Mar;16(3):141-60.
- Gevariya HB, Gami S, Patel N. Formulation and characterization of levofloxacin-loaded biodegradable nanoparticles. Asian J Pharm. 2011;5(Suppl 1):114-119.
- Siramsetti D, Srinivasa RB. Design and evaluation of zolpidem tartrate matrix tablets for extended release using natural gums and HPMC K100M. J Appl Pharm Sci. 2018;8(7):72-77.
- Rapolu K, et al. Optimization and characterization of gastroretentive floating drug delivery system using Box-Behnken design. Drug Dev Ind Pharm. 2013;39(12):1928-1935.
- Garudaiahgari, et al. Response surface optimization of diltiazem HCl gastric floating matrix tablets. Asian J Pharm. 2020;14(3):422.
- Tak, et al. Preparation and optimization of immediate release/sustained release bilayered tablets of loxoprofen using Box–Behnken design. AAPS Pharm Sci Tech. 2017;18(4):1125-1134.
- ich.org. Q1A(R2) guideline [Internet]. Updated 2003 February 6. Available from:https://database.ich.org/sites/default/files/Q1A(R2) Guideline.pdf.
- Wan LSC, Paul WS, Heng, Wong LF. Relationship between swelling and drug release in a hydrophilic matrix. Drug Dev Ind Pharm. 1993;19(10):1201-1210.


 S.sivaranjani *
S.sivaranjani *
 G.ranjani
G.ranjani






















 10.5281/zenodo.13944224
10.5281/zenodo.13944224