Abstract
Pioglitazone, a thiazolidinedione class drug, has been extensively used in the treatment of type 2 diabetes mellitus due to its insulin-sensitizing effects. Recent studies suggest potential benefits in type 1 diabetes management by preserving beta-cell function and improving insulin sensitivity. However, the conventional oral delivery of pioglitazone is associated with systemic side effects and poor bioavailability. To address these issues, this study aims to formulate and evaluate a pioglitazone-loaded niosomal suspension for targeted and sustained delivery. Niosomes, non-ionic surfactant-based vesicles, were prepared using a thin-film hydration method. Surfactant like Span 60 and cholesterol were used to optimize the niosomal formulation. The formulations were characterized for encapsulation efficiency, particle size, zeta potential, and in vitro drug release profile. The stability of the niosomal suspension was evaluated under different storage conditions. Additionally, in vivo studies were conducted using a type 1 diabetes rat model to assess the pharmacokinetic profile and therapeutic efficacy of the niosomal suspension compared to conventional pioglitazone administration. The optimized niosomal formulation exhibited a high encapsulation efficiency (>80%), with a mean particle size of 120-200 nm and a zeta potential of -30 mV, indicating good stability. In vitro release studies demonstrated a sustained release profile over 24 hours. Stability studies confirmed that the niosomal suspension remained stable at 4°C and room temperature for up to three months. In vivo pharmacokinetic studies revealed a significantly higher bioavailability and prolonged plasma concentration of pioglitazone in the niosomal suspension group compared to the free drug. Furthermore, diabetic rats treated with the niosomal suspension showed improved glycemic control and beta-cell preservation, as evidenced by lower blood glucose levels and histological analysis of pancreatic tissue. The formulated pioglitazone-loaded niosomal suspension offers a promising approach for enhancing the therapeutic efficacy and bioavailability of pioglitazone in type 1 diabetes management. This novel delivery system could potentially reduce the dosing frequency and minimize systemic side effects, improving patient compliance and outcomes. Further clinical studies are warranted to validate these findings and explore the full therapeutic potential of pioglitazone-loaded niosomes in type 1 diabetes treatment.
Keywords
Pioglitazone loade niosomes, novel drug delivery, Type-1 diabetes treatment, niosomal suspension.
Introduction
NOVEL DRUG DELIVERY SYSTEM
Novel drug delivery systems is the new system Recent advances in the understanding of pharmacokinetic & pharmacodynamic behaviour of drug have offer a more rational approach to the development of optimal drug delivery system. the novel drug delivery systems (NDDS) are carriers which maintain the drug concentration in therapeutic range for longer period of time There are several advantages of novel drug delivery systems over conventional drug delivery. Drug delivery methods have been developed to reduce medication deterioration, avoid side effects, increase drug bioavailability, and facilitate drug accumulation in the bio-zone. However, there are no universally effective carriers for targeted and regulated drug delivery. Sustained- or controlled-drug delivery systems provide a predetermined pace of drug action at therapeutically efficacious levels in the bloodstream. Localized drug delivery systems control drug release in the immediate region of the target, either spatially or temporally, at a rate-limiting rate. Rate-preprogrammed drug delivery systems regulate drug molecular diffusion. Targeted drug delivery uses carriers for passive or active targeting, with carriers typically anchored with sensory devices that identify their receptor at the target.
- Carrier based Drug Delivery System:
- Liposomes
- Nanoparticles
- Microspheres
- Niosomes
- Resealed erythrocytes as drug carriers
2. Transdermal Drug Delivery Systems:
- Sonophoresis
- Osmotic pump
- Microencapsulation
NIOSOMES
Niosomes are a novel drug delivery system (NDDS) designed to increase absorption and distribute the active ingredient to the intended site while administering the medication at a controlled rate determined by the body's demands during treatment.[1] Drug targeting is the ability to concentrate a medicinal ingredient at the intended site of action with little or no interaction with surrounding tissue. The goal of the controlled drug delivery system is to give the right pharmaceutical release profile for a longer amount of time. One way to put the concept of a controlled release mechanism into practice is using niosomes.[2] In the 1970s, researchers studying cosmetics were the first to find that non-ionized surfactants might self-assemble into vesicles. Niosomes, or non-ionic surfactant vesicles, are small spherical structures that form when nonionic surfactants from the alkyl or dialkyl polyglycerol ether family are coupled with cholesterol levels.[3] Medication is delivered in vesicles using the niosomal drug delivery technology. The process that creates the vesicle involves combining cholesterol in aqueous settings with a non-ionic surfactant of the alkyl or dialkyl poly glycerol ether type of a bilayer (thus the word niosomes), and then hydrating the mixture. Niosomes are quite small and microscopic in size. These particles have diameters that fall into the nanometric range. Compared to liposomes, which are susceptible to oxidation and damage because of their lipophilic nature, niosomes exhibit greater stability. Niosomal formulations provide a more concentrated effect since they remain in the bloodstream longer due to the non-ionizing surfactants in them.[4,5] The focus has shifted to niosomes due to the limitations of liposomes. Both liposomes and niosomes serve as drug delivery systems for both lipophilic and amphiphilic medications.Phospholipids are quickly degraded because of the existence of an ester bond.[6] cholesterol is essential for the formation of niosomes and hardens the vesicles, high cholesterol levels in the bloodstream can affect medication permeability and penetration as well as fluidity. There are several ways to administer niosome medications, including transdermal, intravenous, oral, ophthalmic, and subcutaneous.[7] Reducing the relative concentration of a medication while concentrating it in the target tissues is the goal of targeted medicine delivery. A number of ideas have been proposed to explain how niosomes may promote drug transfer across the skin.[8] Numerous delivery methods for niosomal medications have been investigated, including as intramuscular, intravenous, peroral, and transdermal injection. Moreover, niosomes have been discovered to function as drug-transporting vesicles that enhance drug absorption across cell membranes, localize in certain organs and tissues, and avoid the reticuloendothelial system.[9]
COMPOSITION OF NIOSOMES
They contain a hollow area in the middle containing hydrophilic and hydrophobic drugs. Span60, an amphiphilic surfactant, is used as a vesicle stability surfactant in conventional niosomal vesicles, maintained by a mixture of cholesterol and anions. [7]
1. Cholesterol
2. Non-ionic surfactants
3. Charged molecule
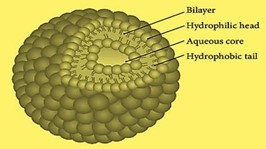
Fig No. 1: Structure of Niosome [10]
Cholesterol is steriod precursor is used to fortify niosome preparations and give niosomes their rigidity and form. Steroids are vital components because they alter the bilayer's permeability and fluidity. Nonionic surfactants frequently include cholesterol, a waxy steroid metabolite, to give them rigidity and orientational order. It is an amphiphilic molecule, with its aliphatic chain facing the hydrocarbon chain of the surfactant and its OH group facing the aqueous phase. [11] Non-ionic Surfactants are commonly employed in the synthesis for niosomes. Surfactants without charged groups in their hydrophilic heads are called nonionic surfactants. They are less toxic, more stable, more biocompatible than their anionic, amphoteric, or cationic counterparts. They are therefore preferred for the in vitro and in vivo production of stable niosomes. Amphiphilic chemicals with two different regions—one hydrophilic (water-soluble) and one hydrophobic (organic soluble)—make up nonionic surfactants.[12]
Charged molecule:
The stability of the vesicles is increased by the introduction of charged groups into the vesicle bilayer. They raise the surface charge density of the vesicles, which reduces aggregation. Because of the repulsive forces of the same charge, they prevent vesicle fusion and increase potential zeta values.However, increasing the quantity of charged molecules can hinder niosome formation.[12]
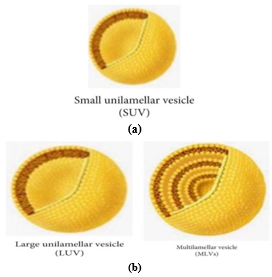
Fig No. 2: Size of niosomes [13]
Multilamellar vesicles (MLVs):
Multilamellar vesicles (MLVs) are vesicles between 100 and 1000 nm in diameter, formed by bilayers adjacent to the aqueous lipid section. They are commonly used due to their ease of preparation, stability, and suitability for lipophilic drugs.[14] Large unilamellar vesicles (LUVs): Membrane lipids, with a high aqueous part to lipid section ratio, can absorb bioactive resources in LUV vesicles with a diameter of 100-250 nm [15] Small unilamellar vesicles (SUVs): Small unilamellar vesicles, ranging from 10 to 100 nm, are produced through processes like sonication, high-pressure homogenization, and extrusion techniques.[16]
ADVANTAGES
-
- A vesicle suspension used based on a water carrier offers better patient compliance than oily dosage formulations.
- Accurate and controlled delivery of drugs.
- Vesicle characteristics can be influenced by variations in concentration, surface charge, tapping volume, size lamellarity, and composition.
- Surfactants can function as a depot formulation, enabling controlled drug release, and they don't need any special circumstances to be stored or transported, such as low temperature or an inert environment.
- They increase the oral bioavailability of poorly soluble medications.
- Despite being in emulsion form, their structure is stable.
- They can use the metabolism of enzymes to protect the drug.
- They can enhance the skin's ability to absorb drugs.[17-20, 7, 10]
DISADVANTAGES
-
- Dissatisfaction on a physical level. Inadequate ability to load drugs.
- Combination Specialized machinery is needed for manufacturing.
- It costs a lot.Medication spills that are confined methods that require a lot of time.
- The dispersion's shelf life is shortened by the hydrolysis of medications in capsules.
- Niosome preparation takes a lot of time. [2,7,10,17,19,20]
MATERIALS:
Pioglitazone, Span 60, Cholesterol, Sorbitol, Citric Acid, Methyl Paraben, Propyl Paraben, Orange Oil, Glycerine, Ethanol, Distilled Water
EXPERIMENTAL WORK
A. PREFORMULATION STUDIES
- Solubility:
Solubility of Pioglitazone tested for water, ethanol and methanol.
- Melting Point:
For melting point determination, we used thiel’s tube determination method.
- Calibration by UV Spectrophotometer:
Weigh 10 mg of Pioglitazone accurately and dissolve it in ethanol to create a 100 mL stock solution. Prepare dilutions of Pioglitazone in ethanol to obtain the required concentrations. (5,10,15,20,25µg/mL) Use ethanol as a blank and fill a quartz cuvette with ethanol. Place the cuvette in a spectrophotometer and zero the instrument at 224 nm. Measure the absorbance of each standard solution at the ?_max and record the absorbance for each concentration.
- IR Spectroscopy of Drug:
The KBr Pellet method involves weighing and grinding a small amount of pioglitazone with 100 mg of KBr to create a homogeneous mixture. The mixture is then transferred into a pellet die and compressed using a hydraulic press to form a thin, transparent pellet. The Bruker IR spectrometer is set up, and the software is initialized. A background spectrum is measured with no sample in place, or using a clean KBr pellet. The sample is placed in the sample holder and the pioglitazone is placed directly onto the ATR crystal. Parameters such as resolution, number of scans, and wavenumber range are set. The data acquisition process begins to collect the IR spectrum of the pioglitazone sample.
B. METHOD OF PREPARATION
1. Formulation of Niosomes
Central Composite Design (CCD) is a statistical technique used for optimizing formulations by studying the effects of multiple factors and their interactions. When formulating niosomes using cholesterol and Span 60 (nonionic surfactant), CCD can help in determining the optimal concentrations of these components to achieve desirable characteristics such as particle size, encapsulation efficiency, and stability. This process involves weighing non-ionic surfactants and cholesterol, a mixture of chloroform and methanol, and pioglitazone in a round-bottom flask. The niosomal suspension is transferred to a sonicator, where it is sonicated for 5-10 minutes to convert MLVs into small SUVs. The temperature is maintained at 40°C during sonication to prevent overheating and drug degradation. The ratio of surfactant to cholesterol can be optimized based on the desired characteristics of the niosomes.
C. CHARECTERIZATION
- Particle Size:
The study analyzed the average vesicle size and potential of niosomal formulations using particle size, entrapment efficiency, and in-vitro release studies. The optimized formulations were measured using Horiba SZ-100 and diluted with HPLC grade water.
- Entrapment Efficiency:
For Entrapment efficiency take solutions into test-tubes which contain 15 ml of ethanol and keep it for ultracentrifugation at 3000 rpm for 1 Hr. After that remove supernatant and calculate the entrapped drug by using UV spectrophotometer.
? = ED/TD×100
Table No.1: Responses of Batches
a) 3D Graph
Fig No. 3: 3D Particle Size
DISCUSSION:
The Fig No. 5 shows that the particle size show regression of given dependable factors the particle size is not affected by concentration of in dependable variable span but it is affected by cholesterol. Fig no 6 shows that plane smooth surface graph and regression value. Entrapment efficiency is dependent on both variables and it increases with both concentration of span and cholesterol.
b) Predicted Vs Actual
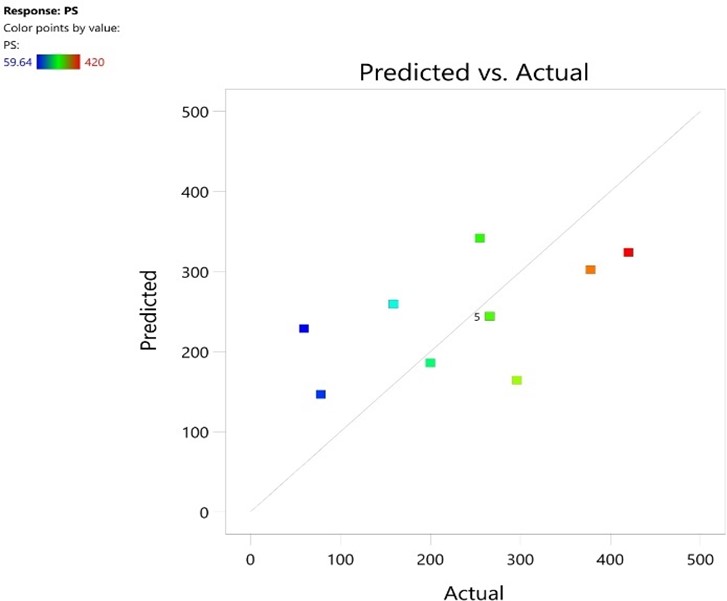
Fig No 5: Particle Size (Pre.vs.Act)
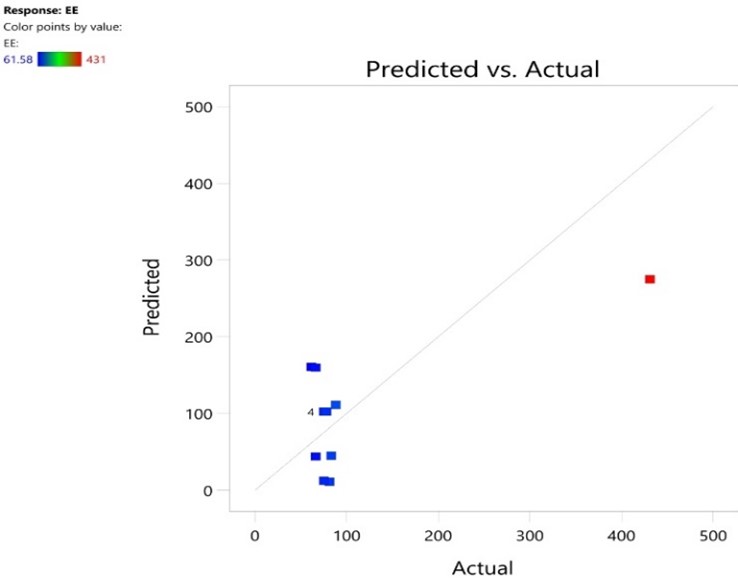
Fig No 6: Entrapment Efficiency
DISCUSSION:
The fig no 7 shows that the values are near to the diagonal line so the actual values are better and simmalar performance with predicted values in case of particle size. The fig no 8 shows 8 points that actual values are come near to the predicted values in case of entrapment efficiency.
c) Counter Plot
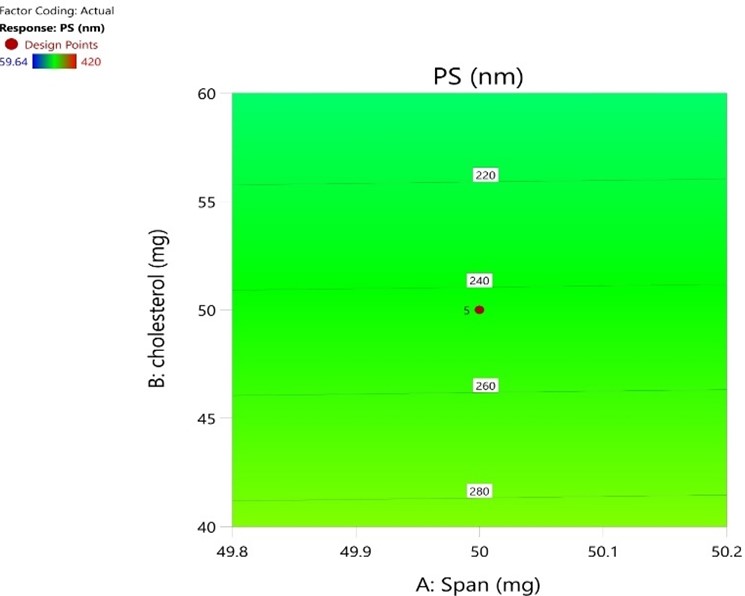
Fig No. 7: Particle Size (CP)
Fig No. 8: Entrapment Efficiency(CP)
DISCUSSION:
The fig no 9 shows that the amount of concentration of cholesterol and span regrets and show similar effect. The fig no 10 show the entrapment efficiency is exactly dependent on the both variables ie. Span and Cholesterol.
d) Interaction
Fig No.9: Particle Size (Interaction)

Fig No.10: Entrapment Efficiency (Interaction)
DISCUSSION:
The fig no 11 of particle size shows negligible interaction. The fig no 12 of entrapment efficiency show little interaction. By the CCD method of formulation F10 is found to be optimum batch because it show high entrapment efficiency and smaller particle size
e) Anova
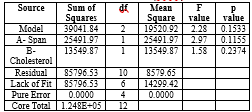
Table No.2: Particle (Anova)
DISCUSSION:
Factor coding is Coded. Sum of squares is Type III - Partial The Model F-value of 2.28 and 15.33% implies the model is significant relative to the noise. P-values less than 0.0500 indicate model terms are significant. In this case there are no significant model terms. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model.
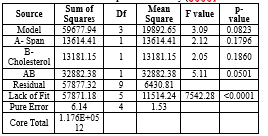
Table No.3: Entrapment Efficiency (Anova)
DISCUSSION:
Factor coding is Coded. Sum of squares is Type III - Partial The Model F-value of 3.09 and 8.23% implies the model is significant relative to the noise. P-values less than 0.0500 indicate model terms are significant. In this case there are no significant model terms. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model. The Lack of Fit F-value of 7542.28 implies the Lack of Fit is significant. There is only a 0.01% chance that a Lack of Fit F-value this large could occur due to noise. Significant lack of fit is bad -- we want the model to fit.
Preparation Of Suspension
Procedure:
To create a pioglitazone-loaded niosomal suspension, measure the necessary amounts of glycerine and sorbitol, dissolve them in distilled water, and heat them gently. Add citric acid and methyl paraben to the solution, and mix thoroughly. Add the niosomal dispersion to the suspension base, stirring continuously to ensure uniform distribution. Add orange oil for a pleasant flavor. Measure the pH of the suspension using a pH meter and adjust it to the desired range using the citric acid solution. Homogenize the suspension using a high-speed homogenizer, and filter the suspension to remove particulates. Transfer the prepared suspension into sterile containers and seal tightly to prevent contamination. The final product will be a clear and clear pioglitazone-loaded niosomal suspension. The process ensures a uniform and stable formulation.
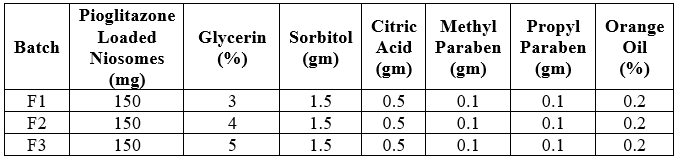
FORMULATION TABLE:
Table No.4: Formulation table of Suspension
Evaluation of Suspension
Sedimentation Method
A graduated cylinder or transparent container is used to observe sedimentation. Pour the suspension into the vessel, ensuring no air bubbles are trapped. Record the initial time (t=0) and observe the process at regular intervals. Use graduations to measure the height of the clear supernatant liquid, note the sedimentation rate, clarity of the supernatant, and any sediment layer formation.
Table No.5: Evaluation table of Suspension
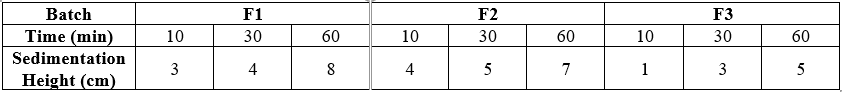
By sedimentation method for evaluation, we found optimum batch F3 because it showing minimum height after 60 min showing that it having fine particle size and maximum stability.
RESULT AND DISCUSSION
Pre-formulation Studies:
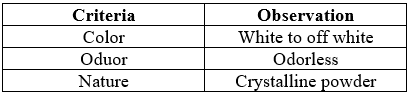
1. Organoleptic Properties
Table No.6: Organoleptic Properties
2. Solubility of Pioglitazone
Pioglitazone is having low solubility in water, but it is moderaDtely soluble in organic solvents like ethanol, methanol.
Table No.7: Solubility

For pioglitazone, the experimentally determined melting point typically falls within a specific range. The melting point of pioglitazone is generally reported to be 184°C.
4. Characterization by UV Spectrophotometer
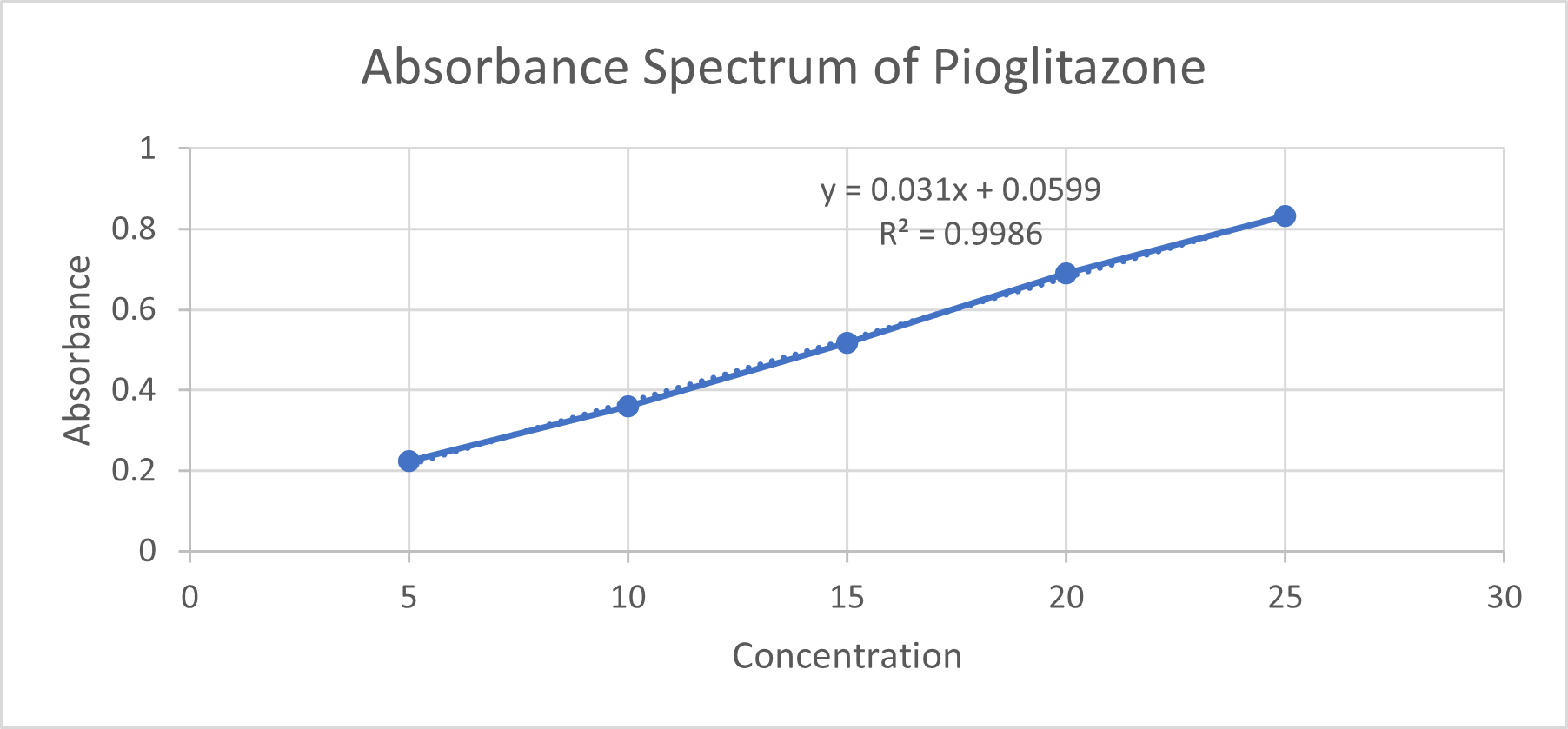
Fig No.11: Absorbance Maxima of Pioglitazone at 224 nm
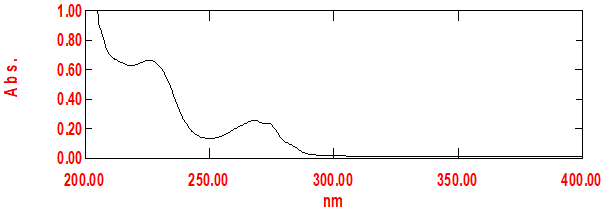
DISCUSSION:
The results of the standard curve for pioglitazone using a UV spectrometer indicate a linear relationship between the concentration of pioglitazone and the absorbance readings at a specific wavelength. The drug had ? max of 272.0 nm.
Table No.8: Calibration of Pioglitazone in Ethanol
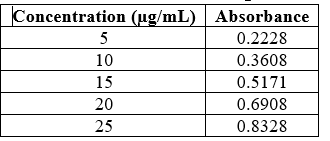
Fig No.12: Calibration Curve of Pioglitazone
5. Characterization By IR
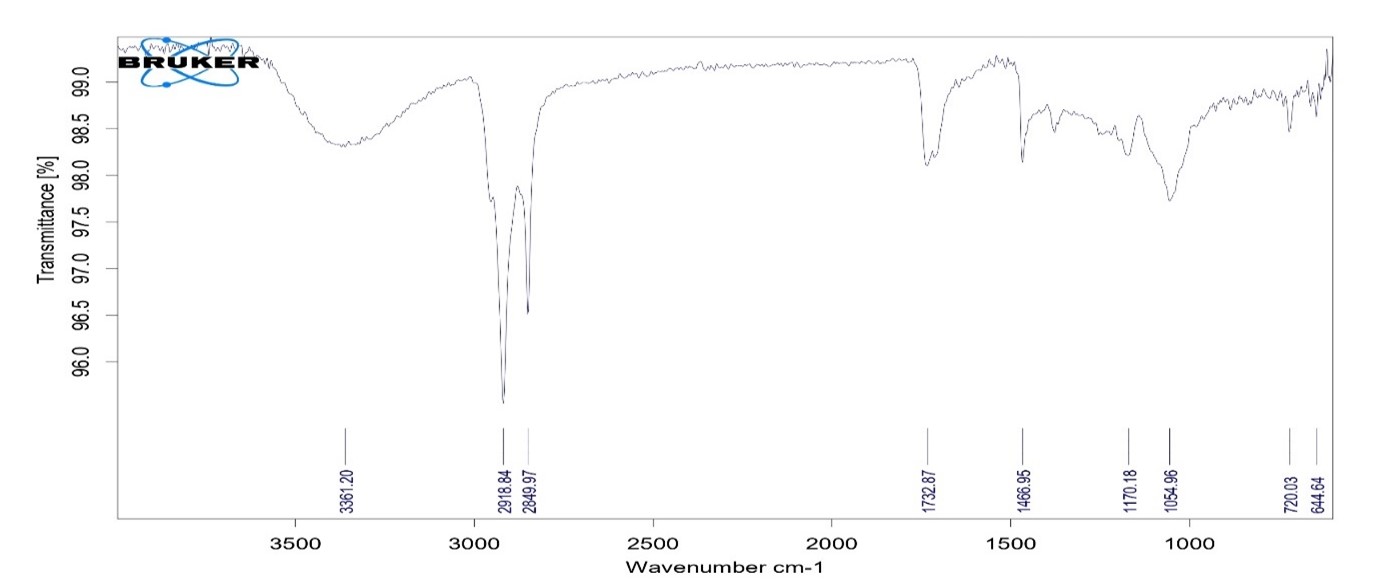
a) Drug FTIR:
The infrared (IR) spectroscopy results of pioglitazone HCL indicate characteristic peaks corresponding to functional groups present in the molecule. Common peaks observed include a broad peak around 3300 cm^-1 corresponding to the N-H stretching vibration, peaks around 1700-1750 cm^-1 indicating the presence of carbonyl groups, and peaks around 1500-1600 cm^-1 corresponding to aromatic C=C stretching vibrations. Additionally, peaks in the fingerprint region (below 1500 cm^-1) provide further structural information. These results confirm the identity and structural features of pioglitazone HCL, aiding in its characterization and analysis.
Table No.9: Interpretation of data of FTIR

Fig No.13: IR of Drug

b) Drug And Polymer FTIR
The infrared (IR) spectroscopy results of the combination of pioglitazone, spasm 60, and cholesterol indicate distinct peaks corresponding to the functional groups present in each compound. Common peaks observed include those for pioglitazone, such as the N-H stretching vibration around 3300 cm^-1, carbonyl groups around 1700-1750 cm^-1, and aromatic C=C stretching vibrations around 1500-1600 cm^-1. Peaks corresponding to spam 60 and cholesterol functional groups will also be present, providing additional information about their chemical structures. These results aid in the characterization and analysis of the mixture, facilitating the identification and quantification of each compound present.
Fig No.14: IR of Drug + Excipient
- Particle Size and Zeta Potential
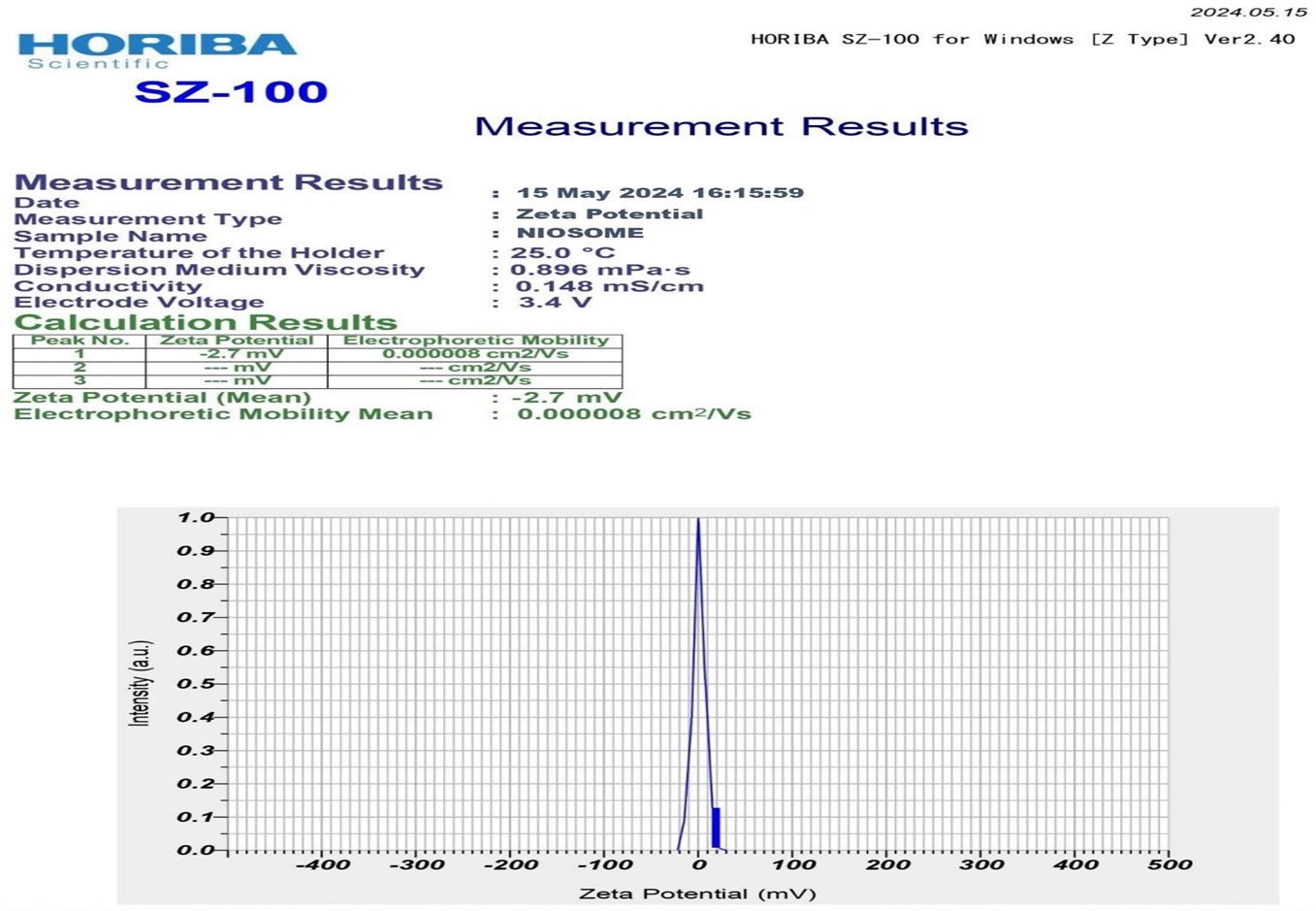
The average particle size of pioglitazone loaded noisome was found to be 230 nm. of optimum batch F10. And The zeta values for niosomal formulations were found to be in range of -15.04 ± 0.45 mV to -31.04 ± 0.25 mv. The zeta potential of the niosome under study was found to be 31.04 ± 0.25 mV as shown in graph. The results revealed that the zeta values of the vesicles increase toward negative with increasing the HLB values of the surfactants. The effect of HLB values of surfactants on zeta potential could be explained in terms of surface energy, which tends to increase with increase in HLB values toward the hydrophilicity. Increase in surface energy of the vesicles.
Fig No.15: Particle size of Optimum Batch
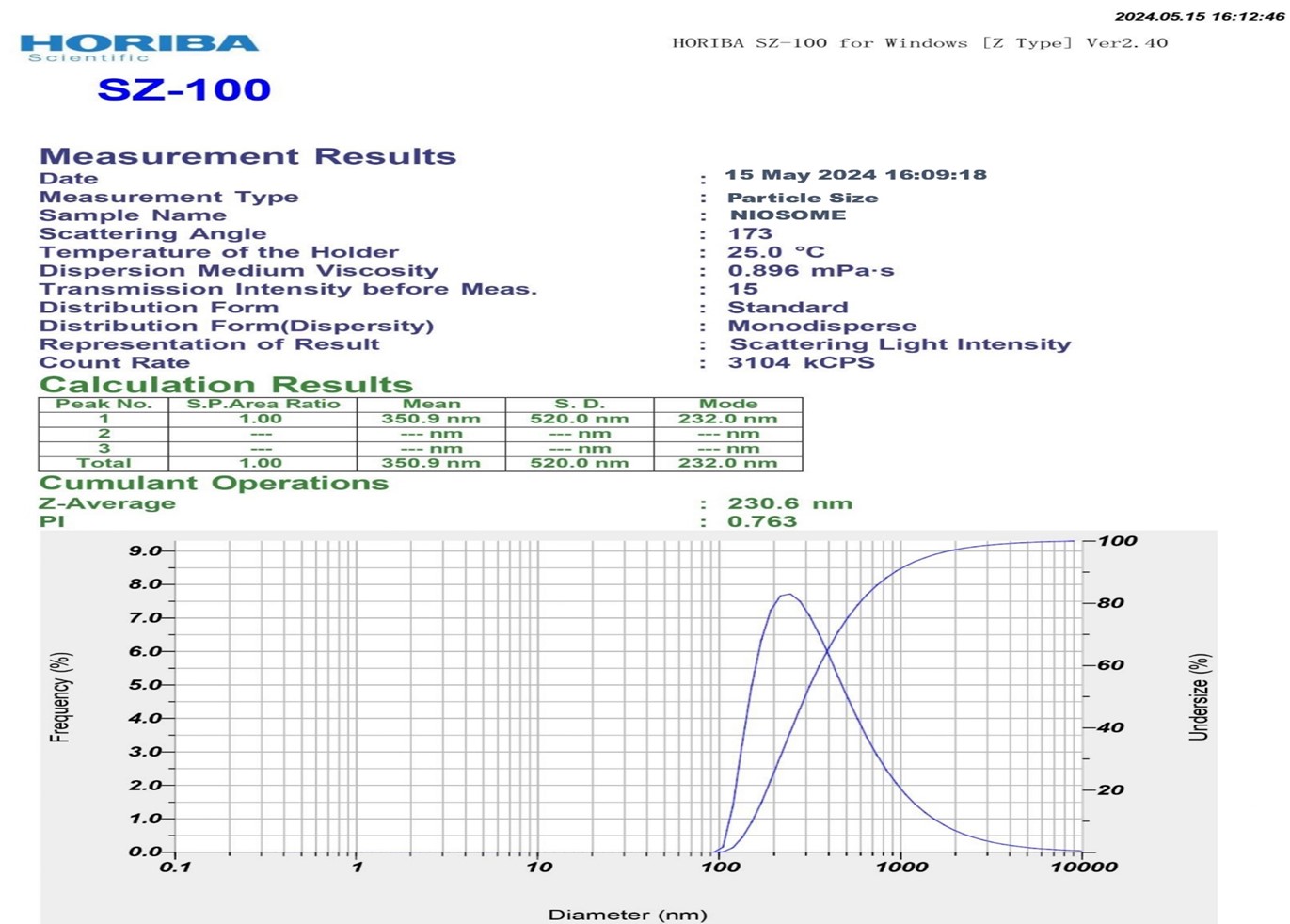
Fig No.16: Zeta Potential of Optimum Batch
7. In-vitro Dissolution Study
Table No.10: Dissolution Study
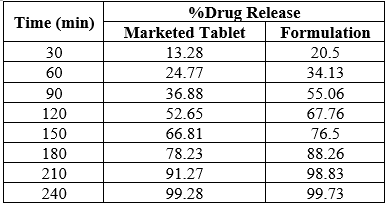
Table No.11 : Kinetics of Dissolution Study
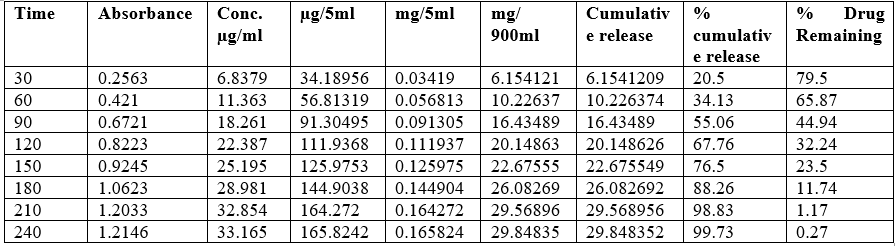
Fig No.18: First order Kinetic
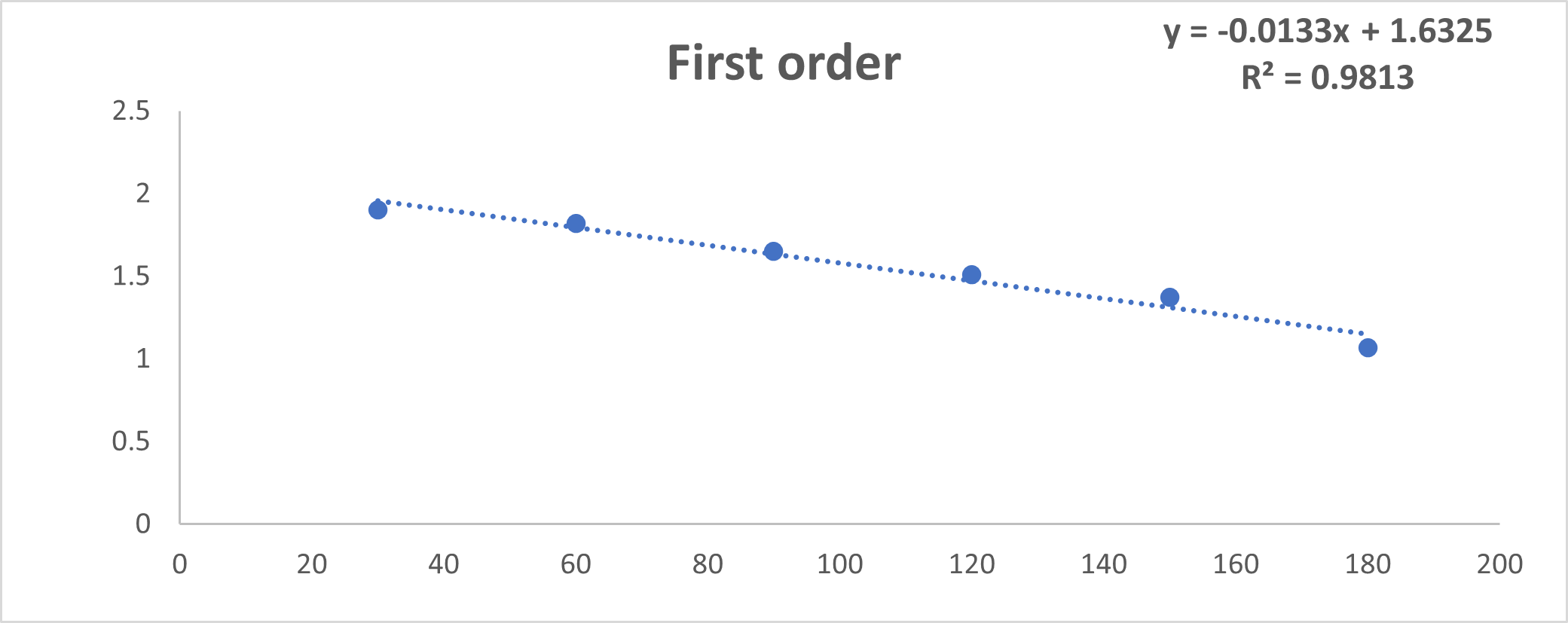
Fig No.19: Higuchi Model
Fig No.20: Zero Oder Kinetics
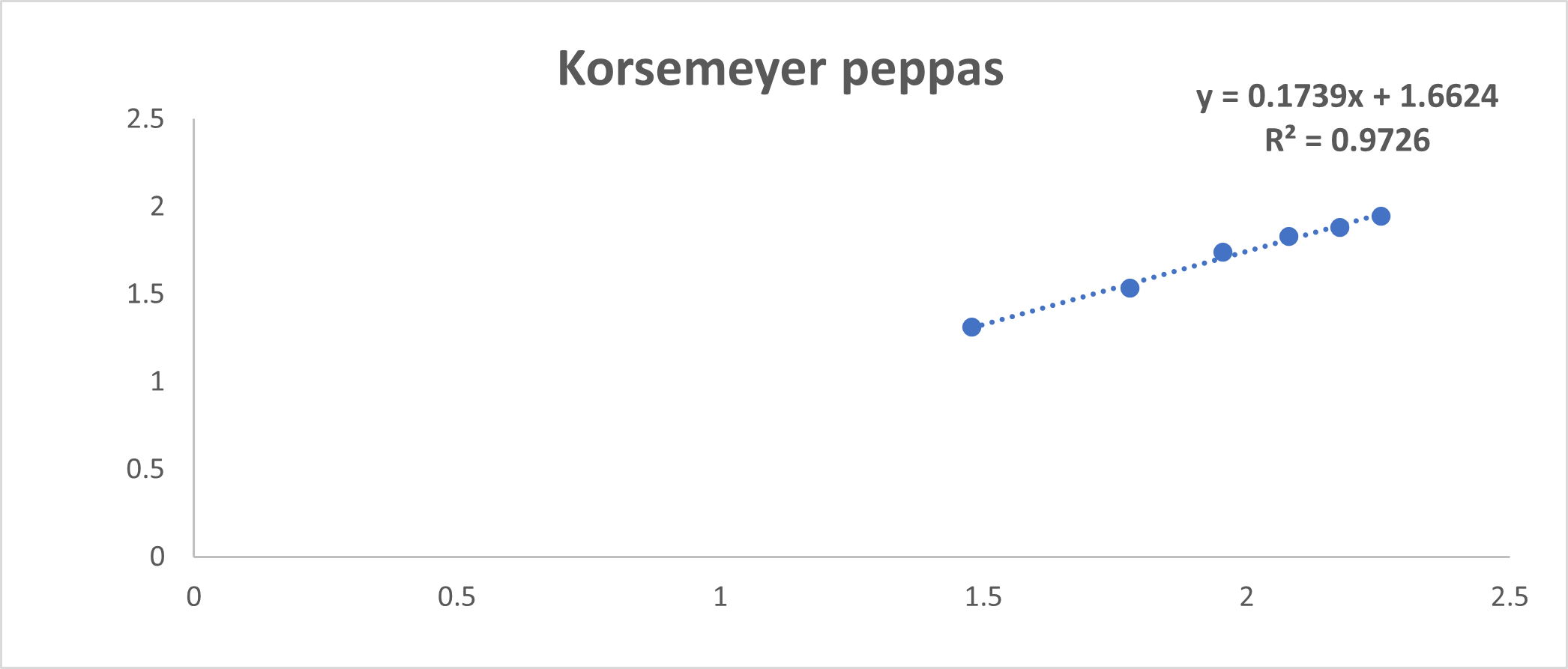
Fig No. 21: Korsemeyer Peppas model
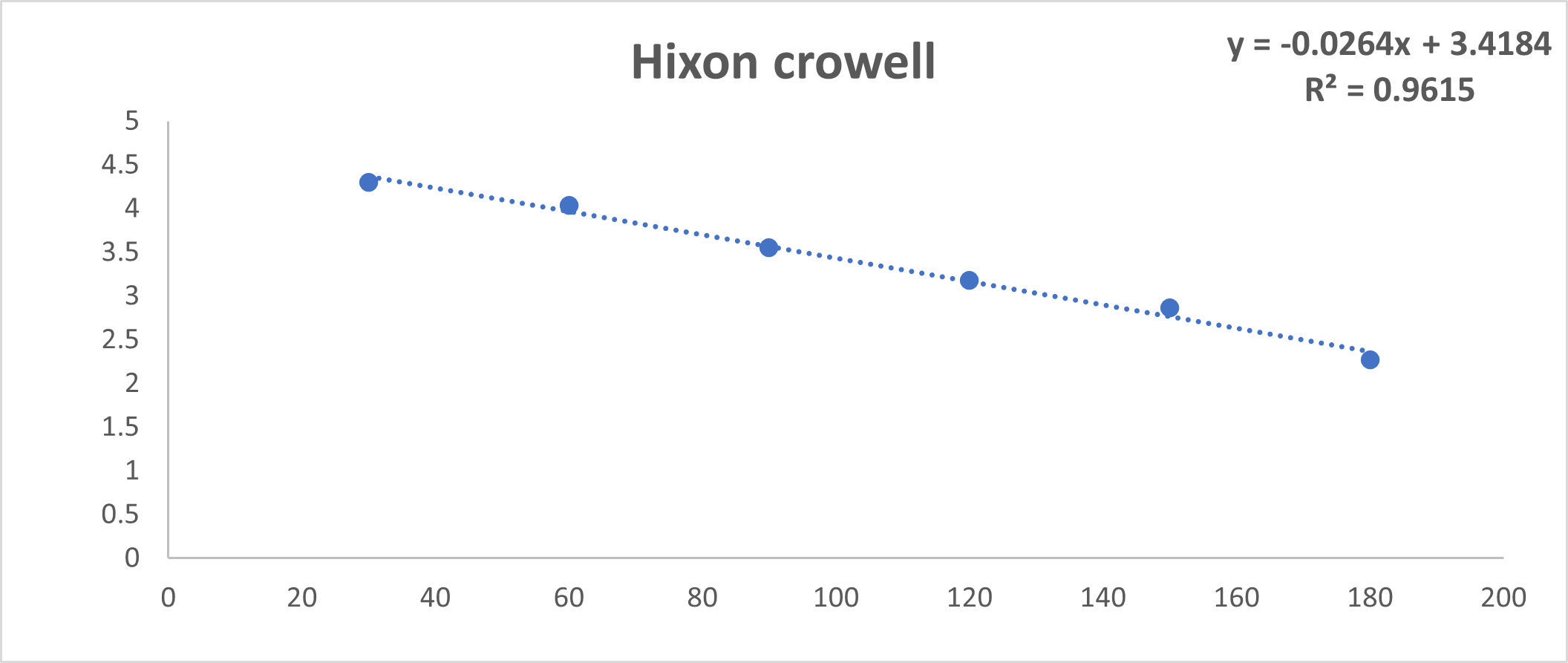
Fig No.22: Hixon Crowell Model

Discussion: By using invitro study and by all the fig we said that formulation follow Higuchi model.
CONCLUSION
In conclusion, the formulation of pioglitazone-loaded niosomal suspension presents a promising avenue for enhancing the therapeutic efficacy of pioglitazone in the management of type 2 diabetes mellitus. The niosomal formulation exhibited favorable physicochemical properties, including uniform size distribution and high encapsulation efficiency, indicating its potential as a stable drug carrier. Moreover, in vitro release studies demonstrated sustained drug release kinetics, suggesting prolonged therapeutic action and improved patient compliance compared to conventional dosage forms. However, further investigations, including preclinical and clinical studies, are necessary to evaluate the safety, efficacy, and pharmacokinetic profile of the pioglitazone-loaded niosomal suspension before clinical translation. Overall, this research contributes valuable insights into the development of novel drug delivery systems for optimizing the treatment of diabetes mellitus.
REFERENCE
-
- Singh D, Upadhyay P, Niosomes: a novel vesicular approach, World J Pharmacy Pharm Sci.2016;5(12):1586-92.
- Dhanvir K, Sandeep K, Niosomes: present scenario and future aspects, Journal of Drug Delivery and Therapeutics (2018):35-43. https://doi.org/10.22270/jddt.v8i5.1886
- Singh U, Hak J, Sharma UK, A Review on Niosomes novel drug delivery system, International journal for multidisciplinary research (ijfmr),2023:1-7.
- Gadhiya P, Shukla S, Modi D, Bharadia P, Niosomes in targeted drug delivery :A review, Int J Pharm Res Scho. 2012; 1(2):59-72.
- Gurjar P, Naik N, Chouksey S, Niosome:A promising pharmaceutical drug delivery, Int J Pharm Analysis, 2014; 2(5):425-31.
- Verma KN, Rai AK, Gulzar A, Singh AP, Niosomes:An approach to current drug delivery:A review, Int J Adv Pharma, 2017; 06(02):41-8.
- Katkale, Akshay, Review on niosomes as novel drug delivery system, World Journal of Pharmaceutical Research, 2022, 1137. 10.20959/wjpr20223-23338
- Moura RBP, Andrade LM, Alonso L, Alonso A, Marreto RN, Taveira SF. Combination of lipid nanoparticles and iontophoresis for enhanced lopinavir skin permeation: impact of electric current on lipid dynamics. Eur J Pharm Sci. 2022;168:106048. doi: 10.1016/j.ejps.2021.106048, PMID 34699938.
- Gandhi A, Sen SO, Paul A, Current trends in niosome as vesicular drug delivery system. Asian J Pharm LifeSci. 2012; 2(2):339-53
- Kandpal R, Joshi A, Kumar K, Rajput V, Chauhan V, An updated review on niosomes: a promising drug carrier, International Journal of Indigenous Herbs and Drugs, 2023; 53-57. https://doi.org/10.46956/ijihd.v8i6.512
- Sankhyan A, Pawar P, Recent trends in noisome as vesicular drug delivery system, J Applied Pharm Sci. 2012; 2(6):20-32.
- Seleci DA, Seleci M, Walter JG, Stah lF, Scheper T, Niosomes as nano particular drug carriers:fundamentals and recent applications, J Nanomaterials, 2016:1-13. https://doi.org/10.1155/2016/7372306
- Domenico F, Jonathan C, Leana R. Nanostructured lipid carrier delivery system, composition, and methods; 2021. p. WO2021. PMID 168573.
- Duong VA, Nguyen TTL, Maeng HJ, Chi SC. Nanostructured lipid carriers containing ondansetron hydrochloride by cold high- pressure homogenization method: preparation, characterization, and pharmacokinetic evaluation. J Drug Deliv Sci Technol. 2019;53. doi: 10.1016/j.jddst.2019.101185, PMID 101185.
- Makoni PA, Wa Kasongo K, Walker RB. Short term stability testing of efavirenz-loaded solid lipid nanoparticle (SLN) and nanostructured lipid carrier (NLC) dispersions. Pharmaceutics. 2019;11(8):397. doi: 10.3390/pharmaceutics11080397, PMID 31398820.
- Upreti T, Senthil V. Nanostructured lipid carrier system for the treatment for skin disease-a review. JSM Nanotechnol Nanomed. 2017;5(3):1059-64.
- Sonule M, Gandhi M, Paralkar S, Dabhade D, Pagar S, Niosomes: novel drug delivery system, Int J Pure App Bio Sci. 2014;2(2):267- 74.
- Parmar RP, Parmar RB, Conceptual aspects of vesicular drug delivery system with special reference to noisome, Asian J Pharm Tech. 2013; 3(2):52-59.
- Chandu VP, Arunachalam A, Jeganath S, Yamini K, Tharangini K, Chaitanya G. Niosomes:A novel drug delivery system, Int J novel trends Pharm Sci. 2012; 2(1):25-31.
- Maurya R, Pathak K, Saxena P, Tiwari J, A review on novel drug delivery system:Niosomes, Asian J Pharm Res Dev 2013; 1(4):51- 59.


 Namrata Satkar* 1
Namrata Satkar* 1
 Samrudh Shunde 2
Samrudh Shunde 2
 Pranjal Chougule 3
Pranjal Chougule 3





























 10.5281/zenodo.13117460
10.5281/zenodo.13117460