Abstract
The detection of solifenacin succinate was successfully achieved by developing and validating a new, isocratic, rapid, specific, sensitive reversed phase high-performance liquid chromatographic (RP-HPLC) method. A stationary phase of the HiQ Sil C18 (250 x 4.6 mm) column with a temperature of 25oC was used to quantify solifenacin succinate. The mobile phase ACN: Methanol (85.5:14.5 v/v) at a flow rate of 0.7 mL/min was selected to produce the chromatographic separation of solifenacin succinate, and a photodiode array (PDA) detector operating at 221 nm was used for detection. The linear regression analysis results for plotting the calibration curve showed a good linear relation with r2 = 0.999 with the concentration range of 10–60 µg mL?1. 6.15?g mL?1 was the limit of quantitation (LOQ) and 2.02 ?g mL?1 was the limit of detection (LOD). The developed technique was determined to comply with ICH guidelines for parameters like specificity, linearity, precision, accuracy, limit of detection, and limit of quantification.
Keywords
Reverse phase high-performance liquid chromatography (RP-HPLC), Solifenacin succinate, Method development, Method validation, Chromatogram.
Introduction
The drug of choice for curing overactive bladder syndrome are antimuscarinics, whose benefit ratio is mostly based on how well they select for the various muscarinic receptor subtypes [1]. Muscarinic receptors are essential for several key cholinergically mediated processes, including salivary secretion stimulation and smooth muscle contractions in the bladder. The detrusor muscle is the muscle found in the bladder wall. It is commonly referred to as having an overactive bladder because of uncontrollable spasms of the detrusor muscles. Anticholinergic medications are frequently used in treating overactive bladder syndrome [2]. A competitive muscarinic receptor antagonist of the anticholinergic class, solifenacin succinate, exhibits greater selectivity for the urinary bladder than salivary glands [3, 4]. As compared to tolterodine, oxybutynin, darifenacin, and atropine, solifenacin succinate exhibits higher selectivity for the urinary bladder rather than for salivary glands. As a result, it may be more beneficial symptomatically than currently prescribed anti-muscarinic medications for the treatment of an overactive bladder and dry mouth [5]. M3 receptor is mainly located in the salivary gland and is crucial to the physiological activity of both the bladder and the salivary gland. M2 and M3 receptors are mainly located in the urinary bladder. Solifenacin succinate has a higher affinity for M3 muscarinic receptors and is a novel competitive muscarinic receptor antagonist. While solifenacin may have little effect on binding exocrine M3 muscarinic receptors, it binds to bladder M3 muscarinic receptors with greater affinity than oxybutynin [6, 7]. Solifenacin is almost 98% bound to proteins in human plasma, primarily to ?1-acid glycoprotein. The CYP3A4 isoenzyme is responsible for considerable amounts of solifenacin's hepatic metabolism; yet, there is also evidence of another metabolic route. 4R-hydroxy solifenacin was found in feces. After a chronic dosage, the elimination half-life for solifenacin is roughly 45 to 68 hours [8].
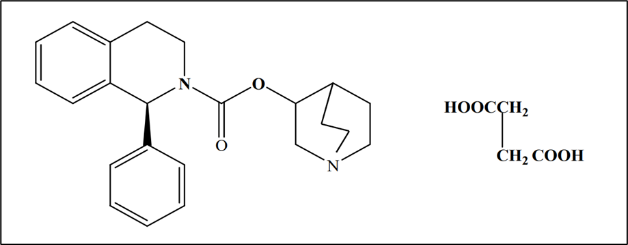
Figure 1. Chemical structure for solifenacin succinate
Solifenacin succinate (figure 1) is also known as butanedioic acid, a molecule with the molecular formula C23H26N2O2 C4H6O4 with a molecular weight of 480.55 g/mol. It is also known as oct-3-yl-3,4-dihydro-1-phenyl-2(1H)-isoquinoline carboxylate (1:1). Solifenacin succinate is available as a crystalline powder or as a white to yellowish-white crystal. It dissolves readily in methanol, glacial acetic acid, acetonitrile, and water at room temperature. Tablets containing solifenacin succinate are designed to be used orally and are available in two dose strengths: 5 mg and 10 mg. This tablet with a dose of 5 mg should be taken once daily. It may be possible to raise the dose to 10 mg once regularly if the dose of 5 mg is well tolerated. It is administered orally with water and should be swallowed entirely. Food can be consumed or not using a solifenacin succinate tablet. [9]
Fig. 2: Steps involved in analytical method development
The aim and objective of this research were to develop a novel, straightforward, enhanced, precise, rapid, and accurate RP-HPLC method for the evaluation of solifenacin succinate in bulk drugs. The developed method has been successfully used to determine validation parameters as per ICH guidelines.
EXPERIMENTAL WORK
Chemicals and reagents
Analytical quality products including HPLC-grade methanol, water and acetonitrile were bought from FINAR Ltd.
Instrumentation
Chromatography was performed on a Jasco-4000 Extrema system equipped with an HPLC pump (PU-4180) and a PDA detector (MD-4915). Data acquisition and integration were performed using ChromNAV software.
Chromatographic conditions
A HiQ Sil C18 column (250 x 4.6 mm, 5 µm) was used for HPLC separation, which was carried out at room temperature. The mobile phase consisted of ACN: Methanol in ratio 85.5:14.5 v/v. UV detection was carried out at 221 nm, and the selected flow rate was 0.7 mL min?1.
Preparation of stock solution
Methanol was used to generate a standard stock solution of solifenacin succinate (1 mg mL?1). By appropriately diluting the stock solution in methanol, a functional stock solution of solifenacin succinate (0.1 mg mL?1) was created.
Linearity
The concentration range for linearity was 10, 20, 30, 40, 50 and 60 µg mL?1 which were obtained through serial dilutions from the working stock solution (100 µg mL?1). Chromatograms were recorded as the chromatographic conditions were tuned, with a volume of 10 µL from each concentration of the solution being injected. Plotting the drug's peak area versus its concentration revealed its patterns.
Precision
By injecting six replicate injections of solifenacin succinate at a standard concentration under the same chromatographic circumstances, precision was assessed, and the percentage RSD was computed. The reproducibility of the developed procedure is shown by the %RSD.
Intermediate precision
In order to evaluate the level of repeatability of the procedure, the assay concentration (50 µg mL?1) was examined on a separate day. The chromatogram was recorded, the test process was repeated six times, and the percentage RSD was computed.
Accuracy
The accuracy of an analytical process is defined as the extent to which the value, which is acknowledged as the conventional true value or accepted reference value, and the value discovered are in agreement. Through recovery studies, accuracy studies were achieved. The pre-analyzed samples were spiked with a known quantity of standard drug, and the drug recovery was estimated. Three degrees of accuracy (80%, 100%, 120%) of the reference concentration were tested. 10 µg mL?1 of the sample was added to a solution, which was then tested under the same chromatographic conditions after being spiked with 80%, 100%, 120% of the reference Solifenacin solution (8, 10, 12 µg mL?1). The drug's peak area was used to compute the recovery percentage.
Robustness
The ability of the method to withstand minor, intentional alterations in chromatographic conditions is measured by its robustness, which was examined by examining the impact of small variations in the wavelength (±0.3), temperature, and flow rate (±0.1 mL/min).
Limit of detection (LOD) and Limit of quantification (LOQ)
The analytical method's LOD refers to its capacity to identify the analyte's lowest concentration and LOQ refers to the lowest concentration of the analyte at which a quantitative analysis may be performed with reasonable accuracy and precision. Based on the signal-to-noise ratio, it can be calculated.
System suitability
The number of theoretical plates, capacity factor, asymmetry factor, and relative standard deviation (RSD) for peak area for repeated injections are some of the system suitability criteria.
Specificity
The drug peak's purity was measured using a PDA detector in order to determine specificity.
Assay method development
The average weight was determined by weighing 10 tablets. An amount equivalent to 10 mg of Solifenacin succinate was weighed and dissolved in methanol. Following the 30 min of sonication the solution was filtered. The filtrate was introduced into the HPLC system after being further diluted to a concentration of 10 µg mL?1.
RESULT AND DISCUSSION
A Reversed Phase High Performance Liquid Chromatographic method was developed and validated to ascertain Solifenacin succinate in tablet dosage forms. A combination of acetonitrile and methanol (85.5:14.5, v/v) was used to provide a satisfactory resolution, and a C18 column (UV detection: 221 nm) (PDA detector) was used.
Optimization of chromatographic conditions
A mobile phase composed of acetonitrile and methanol (50:50, v/v) at a flow rate of 1.0 mL/min was initially used to analyze the samples. Since there was no drug peak under these conditions, the mobile phase was switched to acetonitrile and methanol at ratios of 70:30, 60:40, 85:15, and finally Acetonitrile and Methanol in the ratio of 85.5:14.5, v/v, with a flow rate of 0.7 mL min?1, which produced a sharp peak. The retention time of 4.53 min for solifenacin succinate with a run time of 9 min was reported. The NTP and symmetry factor were 9162 and 1.51, respectively. In Figure 2, the standard chromatogram is shown.
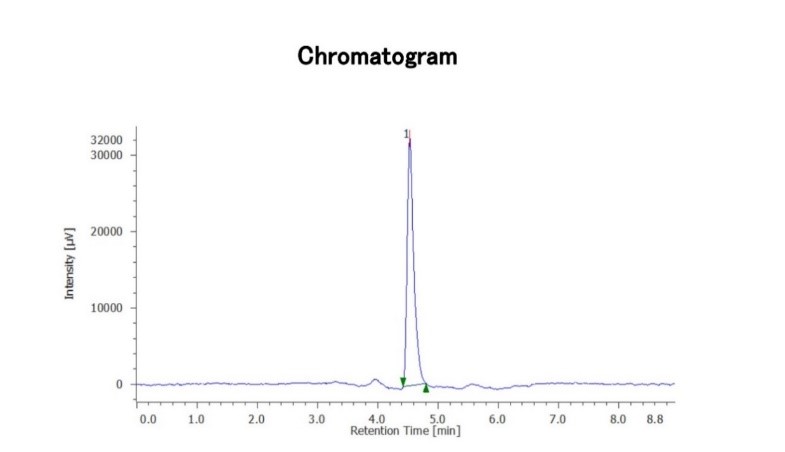
Figure 2. Chromatogram of solifenacin succinate
Validation of the proposed method
The suggested technique was validated in compliance with the International Conference on Harmonization's (ICH) requirements.
Linearity
The test solutions for calculating the linearity of solifenacin succinate (10–60 µg mL?1) were prepared from the stock solution of 100 µg mL?1at various concentration ranges. The peak areas were plotted against the corresponding concentration for constructing the calibration curve. The calibration curve's slope, y-intercept, and correlation coefficient were determined. The calibration curve for solifenacin is shown in Figure 3, and the correlation coefficient was determined to be 0.999.
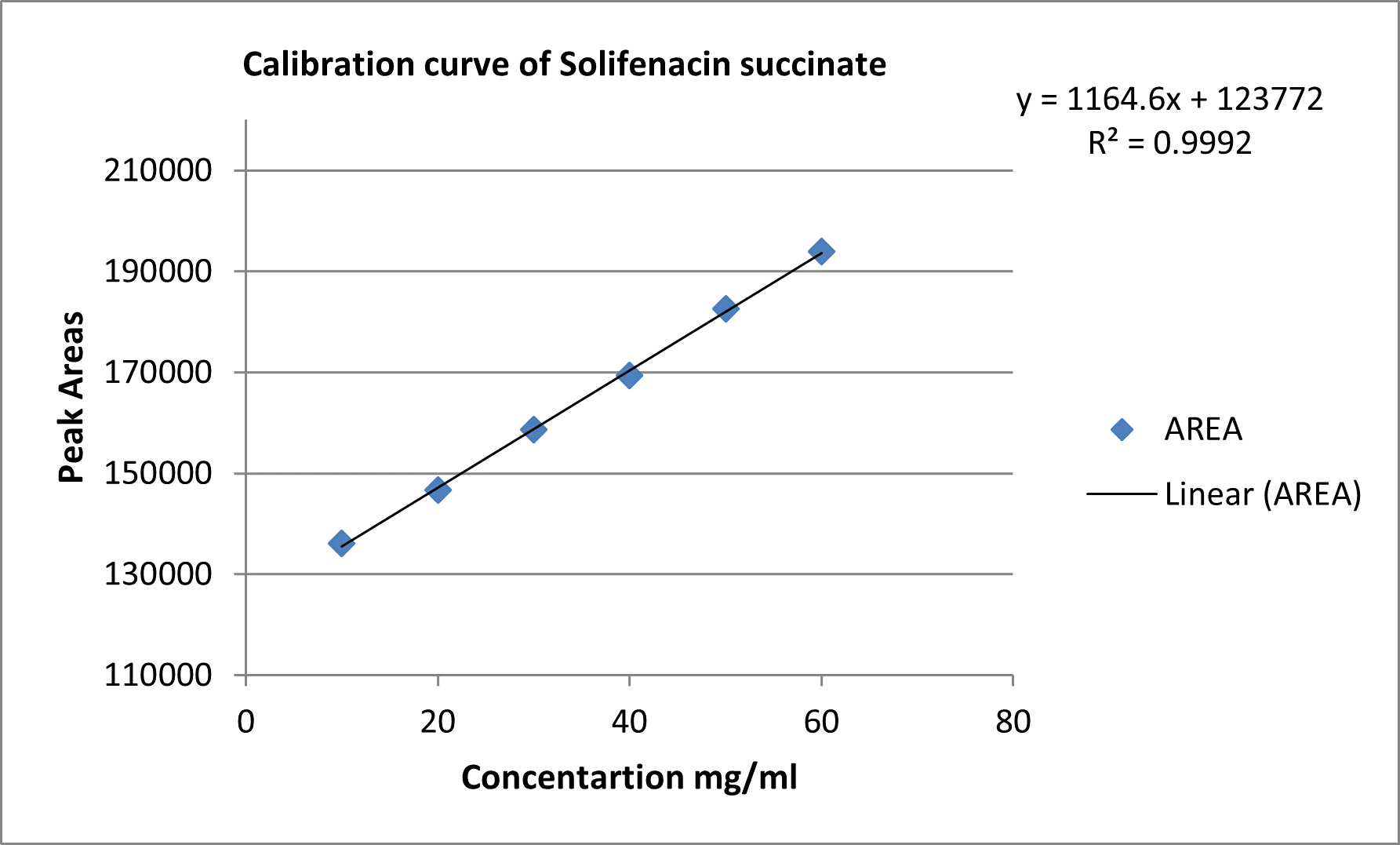
Figure. Calibration curve of solifenacin succinate
Precision
Six replicate solutions of solifenacin succinate of a standard concentration of 50 µg mL?1 were injected under the same chromatographic conditions to determine the precision, and the %RSD was computed. The reproducibility of the developed procedure is shown by the %RSD. It was discovered that the solifenacin’s %RSD was 1.51. Table 3 presents the results of the analysis.
Intermediate precision
In order to evaluate the level of repeatability of the procedure, assay concentration (50 µg mL?1) was examined on a separate day. The chromatogram was recorded, the test process was repeated six times, and the percentage RSD was computed. It was discovered that the solifenacin’s %RSD was 1.40. Table 3 presents the results of the analysis.
Table 1 Precision data of solifenacin succinate
|
Sr.no.
|
Concentration
(?g/ml)
|
Peak Area
|
|
Intraday precision
|
Interday Precision
|
|
1.
|
50
|
231196
|
232170
|
|
2.
|
50
|
232069
|
239042
|
|
3.
|
50
|
234121
|
231265
|
|
4.
|
50
|
231276
|
238171
|
|
5.
|
50
|
238269
|
234521
|
|
6.
|
50
|
239145
|
232570
|
|
Mean
|
234346
|
234623.2
|
|
SD
|
3549.74
|
3275.19
|
|
% RSD
|
1.51
|
1.40
|
Accuracy
The test solution was treated with a known standard quantity (80,100,120%), and the amount of recovered drug was reported. Three concentrations of the Solifenacin standard 8, 10, and 12 µg mL?1 were used to calculate the accuracy. According to ICH criteria, the % recoveries of three different concentrations were found to be between 98 and 102%. The research results showed that solifenacin was recovered above 98% in three distinct concentrations. The outcome is shown in Table 2.
Table 2 Accuracy data of solifenacin succinate
|
Level
|
Mean Peak Area
|
% Recovery
|
Standard Deviation
|
% Relative Standard Deviation
|
|
80%
|
144687.33
|
99.48
|
1.13
|
1.14
|
|
100%
|
147049
|
99.87
|
1.54
|
1.55
|
|
120%
|
149377.67
|
99.88
|
1.56
|
1.56
|
Robustness
The ability of the method to withstand minor, intentional alterations in chromatographic conditions is measured by its robustness, which was studied by examining the impact of small variations in the wavelength (±0.3), temperature, and flow rate (±0.1 mL/min). The symmetry factor and number of theoretical plates were observed to be within limits. Therefore, the method was found to be robust. The results are shown below in Table 3.
Table 3 Robustness data of solifenacin succinate
|
Parameters & Conditions
|
Mean Peak Area
|
Standard Deviation
|
%RSD
|
|
Flow Rate
|
0.6
|
136361
|
941.53
|
0.69
|
|
0.8
|
194171
|
993.24
|
0.51
|
|
Temperature
|
20
|
159809
|
2106.40
|
1.32
|
|
30
|
161984
|
642.67
|
0.40
|
|
Wavelength
|
218
|
230359
|
1952.61
|
0.85
|
|
224
|
161976
|
903.48
|
0.56
|
Limit of detection (LOD) and Limit of quantification (LOQ):
The analytical method's LOD and LOQ were examined based on the signal-to-noise ratio as reported in ICH guidelines. The values of limit of detection (LOD) and limit of quantification (LOQ) were reported to be 2.02 µg mL?1 and 6.15 µg mL?1.
System suitability
The number of theoretical plates, capacity factor, asymmetry factor, and relative standard deviation (RSD) are the system suitability parameters that were found to be within limits as per ICH guidelines.
Assay method development
The average weight was determined by weighing 10 tablets. An amount equivalent to 10 mg of Solifenacin succinate which is 290 mg was dissolved in methanol. Following the 30 min of sonication the solution was filtered. The filtrate was introduced into the HPLC system after being further diluted to a concentration of 10 µg mL?1.
CONCLUSION
For the estimation studies of solifenacin succinate in both bulk drug and their tablet dosage form, a reversed phase HPLC isocratic approach has been established. For the quantification of solifenacin succinate in tablets, the HPLC technique was validated in accordance with ICH guidelines criteria concerning specificity, accuracy, precision, linearity, limit of detection, and limit of quantitation. The precision was exemplified by a relative standard deviation of less than 2 %. A good linear relationship was observed for the drug which is 0.999. Accuracy studies revealed that the recoveries were within limits, indicative of an accurate method. Hence, it can be reported that the reverse phase isocratic HPLC method that has been developed is linear, accurate, rapid, precise, and selective.
REFERENCES
- Francisco J. Solifenacin Pharmacology. Neurologic and Urodinamic Urology. 2010; 63(1): 43-52.
- Wein AJ. Pharmacological agents for the treatment of urinary incontinence due to overactive bladder. Expert Opinion on Investigational Drugs. 2001; 10(1): 65-83.
- Ikeda K, Kobayashi S, Suzuki M. et. al. M 3 Receptor Antagonism by the Novel Antimuscarinic Agent Solifenacin in the Urinary Bladder and Salivary Gland. Naunyn-Schmiedeberg’s archives of pharmacology. 2002; 366 (2): 97–103.
- Michel M C, Oelke M, Zinner N. Novel Muscarinic Antagonists to Treat Incontinence And/or Overactive Bladder. Drug discovery today: Therapeutic Strategies. 2005; 2 (1): 1-6.
- Ohtake A, Ukai M, Hatanaka T.et. al. In Vitro and in Vivo Tissue Selectivity Profile of Solifenacin Succinate (YM905) for Urinary Bladder over Salivary Gland in Rats. European journal of pharmacology. 2004; 492(2-3): 243–250.
- Ito Y, Oyunzul L, Yoshida A. et. al. Comparison of Muscarinic Receptor Selectivity of Solifenacin and Oxybutynin in the Bladder and Submandibular Gland of Muscarinic Receptor Knockout Mice. European journal of pharmacology. 2009; 615(1-3): 201–206.
- Suzuki M, Ohtake A, Yoshino T. et. al. Effects of Solifenacin Succinate (YM905) on Detrusor Overactivity in Conscious Cerebral Infarcted Rats. European journal of pharmacology. 2005; 512 (1): 61–66.
- Product Portfolio | Astellas Pharma Canada, Inc. [Internet]. Astellas.com. 2022; [cited 2024 Aug 21]. Available from: https://www.astellas.com/ca/en/innovation/product-portfolio
- Rami Reddy B V, Srinivasa Reddy B, Subhash Reddy K. et. al. Development and Validation of a Specific Stability Indicating High Performance Liquid Chromatographic Methods for Related Compounds and Assay of Solifenacin Succinate. Journal of Chemistry. 2013; 1–10.


 Vaishali Jadhav*
Vaishali Jadhav*




 10.5281/zenodo.14397385
10.5281/zenodo.14397385