Abstract
The most researched nanocarriers for targeted drug delivery systems are liposomes. Emulsification produces spherical lipid vesicles, or nanoliposomes, with a typical particle size of 50–500 nm. It contains one or more lipid bilayers and is made up of natural or artificial lipids in an aqueous medium. Owing to their high drug loading efficiency, bioavailability, chemical stability, ease of synthesis, biocompatibility, and safe additions, nanoliposomes are frequently employed fine particles in nanomedicine. Their structural resemblance to biological membranes allows them to effortlessly traverse skin membranes and deliver medications to the intended location. Polymer serves as the primary structural component of the transdermal drug delivery system, regulating the drug's release from the formulation. This review article involves detailed information regarding nanoliposomes and transdermal patches including their methods of preparation, loading of active substances and evaluation parameters.
Keywords
Nanoliposomes, Transdermal Patches, Polymers, Phospholipids, Rate-controlling membrane, Elastomers.
Introduction
The nanometric variant of liposomes, known as nanoliposomes, are among the most widely used encapsulation and controlled release technologies. The Greek terms lipos (fat) and soma (body or structure) are the source of the word liposome, which refers to a structure in which internal aqueous compartments are encased in a fatty envelope.(1) Since liposome is a general term covering many classes of vesicles whose diameters range from tens of nanometres to several micrometres, the term nanoliposome has recently been introduced to exclusively refer to nanoscale bilayer lipid vesicles.(2) Liposomes and nanoliposomes are similar in that they have similar chemical, structural, and thermodynamic characteristics but nanoliposomes have a larger surface area than liposomes and can potentially improve controlled release, increase solubility, improve bioavailability, and allow more precise targeting of the encapsulated material. Bilayer lipid vesicles, or liposomes, are excellent models of both cells and biomembranes.(3) Due to their similarity to biological membranes, they are a perfect system for studying both modern biomembranes and the origins, development, and evolution of early cell membranes.(4,5) Liposomes are made up of one or more phospholipid and/or concentric or non-concentric lipid bilayers, and their structural makeup may also include other molecules like proteins. Regarding the quantity of bilayers they hold, they can be single or multilamellar. In their aqueous and/or lipid compartments, they can hold hydrophilic, lipophilic, and amphiphilic compounds.(6) It can function as a TDDS system drug carrier. An additional method of delivering medications through the skin layer is transdermal drug delivery. Before the medication reaches its target location, it passes through the skin and enters the bloodstream, where it circulates throughout the body. (7) A transdermal patch is a medicated patch that can be applied topically to administer medication at a prescribed rate directly into the bloodstream through the layers of skin. Actually, the most practical way to administer is via patches.(8,9) Transdermal patches have advantages over traditional oral dosage forms, including painless and simple administration of therapeutic drug levels and a lower frequency of doses. Since they are non-invasive, patients can stop the treatment at any time during the course of several days. It is more difficult for drug molecules bigger than 500 Daltons to pass through the stratum corneum, and the drug's therapeutic dose should ideally be less than 10 mg daily.(10)
MATERIAL AND METHODS
Materials:
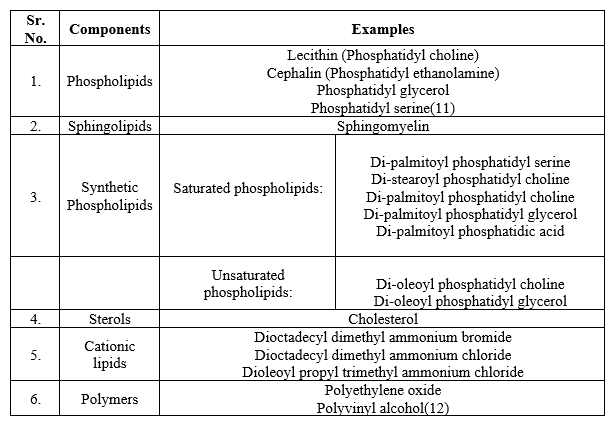
Structural components of nanoliposomes:
Transdermal patch:
Components of transdermal patches:

Methods of Preparation:
Preparation of liposomes
Liposomes can be prepared by three types of processes which are as follow:
1. Mechanical method
2. Replacement of organic solvent
3. Size transformation
1. Mechanical method:
Traditional methods
Bangham method/Thin Film Method: It is the method most frequently used to create traditional nanoliposomes.(18)
Thin film method can be classified into Two types:
- Hand shaking Lipid film hydration
- Non-hand shaking/ Freeze drying Lipid film hydration(19)
Procedure:
The hydrophobic medications are dissolved in a mixture of phospholipids in a polar solvent (such as ethanol). After that, the solvent is evaporated above the phospholipid transition temperature (either using a sample concentrator or a rotary evaporator). After that, a film forms at the flask's bottom and is dried for at least 24 hours under a vacuum desiccator to get rid of any remaining organic solvents before hydration. In the presence of distilled water or a buffer solution, such as phosphate buffer saline (PBS), the hydration is done while stirring. To reduce the size of the vesicles and homogenize the sample, the mixture is then sonicated using a bath or probe sonicator.(20)
Ultrasonic method
This method is exclusively used for production of Small Unilamellar vesicles (SUVs) from Multilamellar Large Vesicles (MLVs).
Instrument used:
Sonicator(21)
The instrument used in ultrasonic method is known as sonicator which can be classified into two types based on sonication techniques.
Two types of sonicator:
- Probe sonicator
- Bath sonicator(22)
Probe sonicator:
This method is employed to reduce size of liposomes. A sonicator tip is submerged in the liposome solution while using the probe sonication method, which is typically utilized for small volumes. To prevent the high energy delivered by the tip from causing a local warming and degradation of the lipidic solution, the bath vessel is submerged in a water/ice bath.
Bath sonicator:
A bath sonicator is used to hold the liposome dispersion in a cylinder. Usually, the lipid dispersion's temperature can be controlled. Compared to sonication by direct dispersal using the tip, this method is easier. In contrast to probe units, the material being sonicated can be shielded in a sterile vessel or in an inert environment.(23)
Replacement of organic solvent
Ethanol Injection Method
The founders of this method are Batzri and Korn (1973). Phospholipids in an ethanol solution are injected into an aqueous phase under carefully monitored circumstances that take into account the pump flow rate, stirring force, and injection temperature (above the lipid transition temperature). After that, to eliminate any remaining solvent traces, the solution is kept under mechanical stirring on a magnetic stirrer or by rotary evaporation at room temperature under low pressure.(24)
Reverse phase evaporation
After adding the lipid mixture to a round-bottom flask, rotary evaporator removes the solvent at a lower pressure. Nitrogen is introduced into the system, causing the lipids to re-dissolve in the organic phase, which is where the reverse phase vesicle forms. The typical solvents used are isopropyl ether and diethyl ether. The solvent is extracted from an emulsion by evaporating it to a semisolid gel under reduced pressure after the lipids have been redissolved. Then, the non-encapsulated material is eliminated. The reverse phase evaporation vesicles (REV) that are produced are liposomes. This technique has the capacity to encapsulate large macromolecules with high efficiency and is used to prepare large uni-lamellar and oligo-lamellar vesicles.(25)
Size Transformation
Dehydration- Rehydration method
This method involves filling an empty buffer with SUVs, rehydrating it with an aqueous solution containing the material to be trapped and then drying the buffers. As a result, solid lipids become dispersed and finely divided. The preferred approach usually involves freeze drying. After that, the vesicles are rehydrated to produce liposomes.(26)
Freeze Thaw Extrusion method
The traditional DRV method is expanded upon by the freeze-thaw technique. The solute to be trapped is vortexed with liposomes created using the film method until the entire film is suspended. The resulting MLVs are then frozen in lukewarm water and vortexed once more. The sample is extruded three times following two cycles of freeze-thaw and vortexing.(27) Eight more extrusions and six freeze-thaw cycles come next. By using the extrusion technique, this process ruptures and defuses SUVs, allowing the solute to equilibrate between the inside and outside. The liposomes then fuse and grow larger, forming Large Uni lamellar vesicles (LUVET). This technique is frequently used to encapsulate proteins.(28)
Drug Loading in Liposomes
Drug loading into liposomes can be accomplished through passive or active methods. When the lipid bilayer forms, hydrophilic drugs are trapped by passive loading in the aqueous core of the liposomes, while hydrophobic drugs build up in the smaller hydrophobic lipid bilayer. (29) In order to guarantee high encapsulation efficiency of valuable chemotherapeutic agents, active or remote loading has been developed. By using a pH gradient and/or possible ionic differences across liposomal bilayer membranes, remote loading into preformed liposomes can be accomplished.(30)
Evaluation of liposomal preparation
Liposomes are evaluated for physical and chemical properties as they influence the Invivo properties of a formulation.
Physical Properties:
Particle size: Particle size can be determined by electron microscopy and laser light scattering.
Surface Charge:
Free-flow electrophoresis of MLVs is the foundation of the technique used to measure surface charge. It utilizes the use of cellulose ester plate dipped in sodium borate buffer with 8.8 pH value. The plate is covered with approximately moles of lipid samples, and it is electrophoresed for 30 minutes at 4 °C. Depending on how charged their surface is, the liposomes split. (31)
Present Drug Encapsulated:
The amount of drug contained in the liposomes aids in estimating how the medication will behave in a biological system.
First, the percentage of drug encapsulation is calculated by separating the drug fraction that is encapsulated from the free drug friction. (32) Next, using the appropriate detergents, the liposome-free encapsulated drug fraction is added to an aqueous solution.
The following techniques are intended to isolate the free drug from the sample:
- Mini column centrifugation method
- Protamine aggregates method(33)
Entrapment efficacy:
Drug that will be entrapped in the liposomes will be measured after the unentrapped was removed. Centrifugation will be used to separate the drug that will not be entrapped by using a cooling centrifuge for ten minutes at 10,000 rpm and 4?C.(34) The supernatant will be removed and the pellet of liposomes will be washed with 5 ml buffer to remove any unentrapped drug. The washing will be combined with supernatant and will be analysed for plant content to calculate the entrapment efficiency.(35)
Chemical properties:
Determination of Phospholipid content:
The evaluation of chemical properties of liposomes includes determination of phospholipid content. The assays known as the Bartlett and Steward assays are frequently used to directly determine the amount of phospholipid present in liposomes.(36)
Preparation of liposomal transdermal patch
Preparation of backing membrane
An aqueous solution of 4%w/v polyvinyl alcohol (PVA) will be used to make the backing membrane. 4 gm of PVA will be mixed into 100 ml of warm, distilled water to create a homogeneous solution with continuous stirring and brief heating at 60 °C. After that, 15 mL of the homogeneous solution will be poured into a glass Petri dish of 63.5 cm2 and dried in a hot air oven at 60 ° C for 6 h.(37)
Formulation of transdermal patches
Using various polymers (HPMC: PVP), transdermal films containing plant extract loaded liposomal preparation will be cast on a petri dish using a solvent evaporation method. The ratios of polymer to polymer and drug will be fixed at 1:1, 1:2, and 2:1, respectively. In each formulation, different concentrations of PVP and HPMC will be used. (38) Propylene glycol and N-dibutyl phthalate will be employed as plasticizers. In each formulation, 1% DMSO will be utilized as a permeation enhancer.(8,39)
Evaluation of transdermal patch
Adhesive property
According to the standardized testing procedures, a patch strip must be applied to a rigid standard test plate (adherend plate), which is typically made of stainless steel, and a specific amount of pressure must be applied to ensure the contact. The strip is then removed from the plate at a predetermined time at a specific angle (180 or 90 degrees) and speed (300 mm/min). Then the adhesion results are observed. (40)
Folding endurance
A specific (2 by 2 cm) section of the strip will be cut consistently, then folded repeatedly until it broke. The number of times the film will be folded at the same spot—either until it broke or developed noticeable cracks—will be used to calculate the folding endurance value.(41)
Thickness
Using a digital micrometer screw gauge, the transdermal patches' thickness will be measured three times, and the mean value and standard deviation will be computed.(42)
Drug content
After dissolving a transdermal patch measuring 2 by 2 cm in 100 millilitres of methanol, it will be shaken constantly for a full day. We will then ultrasonicated the entire solution for fifteen minutes. Following filtration and spectrophotometry. (43)
Percentage moisture content
After each prepared transdermal film will be weighed, it will be kept at room temperature for 24 hours in a desiccator filled with fused calcium chloride. The films will be reweighed after 24 hours, and the percentage moisture content will be calculated using the formula below.(44)
Percentage moisture uptake
Each of the ready-to-use transdermal films will be weighed and kept in a desiccator with a fused saturated potassium chloride solution to sustain 84% relative humidity for a full day at room temperature. The films will be reweighed after 24 hours, and the percentage moisture uptake will be computed using the formula below.(45)
Tensile strength
A tensiometer will be used to assess the patch's tensile strength. It consists of two load cell grips. While the upper one will be movable, the lower one will be fixed.
Two by two-centimetre film strips were positioned in between the cell grips, and force will be gradually applied until the film broke. The dial's reading in kilograms will be used to calculate the tensile strength.(46)
Invitro drug release testing
Drug release testing
For the in vitro drug release tests, a Franz diffusion cell with a receptor compartment capacity of 60 ml will be employed. Using a cellulose acetate membrane from the transdermal matrix-style patches that will be prepared, the medication will be identified. The donor and receptor compartments of the diffusion cell will be divided by a cellulose acetate membrane with a pore size of 0.45 ?. (47) The cellulose acetate membrane will be mounted with the transdermal patch ready to use, and sealed with aluminium foil. The receptor compartment of the diffusion cell will be filled with 7.4 pH phosphate buffer. The entire assembly will be placed on a hot plate magnetic stirrer, and throughout the experiments, magnetic beads in the receptor compartment will be used to continuously stir the solution at 50 rpm while maintaining the temperature at 37±0.5 °C, or the average body temperature. (48,49) The samples will be collected at different times and spectrophotometric analysis to determine the presence of drugs. While the experiment, the manual sampling needs ongoing, cautious supervision. Since the receiver compartment's air bubbles can easily enter at the time of sample collection. Every time a sample is removed, the receptor Phosphate buffer in an equivalent volume will be added back into the step.(50)
Drug permeation testing
Using a Franz diffusion cell and the full-thickness abdominal skin of a male Wistar rat weighing 200–250 g, an in vitro permeation study will be conducted. Hair will be meticulously extracted from the area of the abdomen using an electrical shear; the skin's dermal side will be completely cleaned with distilled water to get rid of any residue from cells or vascular structures. It spent an hour in phosphate equilibration. Before starting the experiment, make a saline pH 7 buffer. (51) A heater with a thermostat-controlled setting will keep the cell temperature at 37±0.5 °C. The rat skin fragment will be positioned in the space between the diffusion cell compartments, with its epidermis pointing towards the donor compartment. Periodically, the 1 ml sample volume will be taken out of the receptor compartment at intervals 0.5,1,2,3,4,6,8,10,12, 16, 20, and 24 hours later, and the same volume of new medium will be added. After passing through the Whatman filter, the samples will be studied in the UV spectrophotometer.(50,52)
CONCLUSIONS
Liposomes are best suitable nanocarriers which can easily cross the dermal barriers due to their similar structures. Hence, liposomes are most preferrable nanocarriers for transdermal drug delivery systems. It has been established that liposomes are incredibly effective drug delivery vehicles. There are many different pharmaceutical applications for liposomes. Liposomal medications show decreased toxicity. Additionally, its prolonged circulation residence time and improved drug delivery to the site of the disease are now gaining clinical acceptance. Patches have the ability to deliver continuous drug dosing for extended periods of time by evading the digestive system and first-pass metabolism. Transdermal patches have advantages over traditional oral dosage forms, including painless and simple administration of therapeutic drug levels and a lower frequency of doses. Since they are non-invasive, patients can stop the treatment at any time during the course of several days. This study includes information regarding the formulation of transdermal patches containing nanoliposomes as carrier system to promote targeted and controlled drug delivery.
REFERENCES
- Vishvakrama P, Sharma S. LIPOSOMES: AN OVERVIEW. Journal of Drug Delivery and Therapeutics. 2014 Jun 25;0(0).
- Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Vol. 8, Heliyon. Elsevier Ltd; 2022.
- Vishvakrama P, Sharma S. Liposomes: an overview. Journal of Drug Delivery and Therapeutics. 2014;47–55.
- Manish Kumar, Arpita Singh, Swarnima Pandey, Mohd. Aqil Siddiqui, Nitish Kumar. LIPOSOMES: TYPE, PREAPRATION AND EVALUATION. International Journal of Indigenous Herbs and Drugs. 2021 Feb 15;17–22.
- Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16(7):307–21.
- Pradhan B, Kumar N, Saha S, Roy A. LIPOSOME: METHOD OF PREPARATION, ADVANTAGES, EVALUATION AND ITS APPLICATION [Internet]. Available from: www.japtronline.com
- Tanwar H, Sachdeva R. Transdermal drug delivery system: A review. Int J Pharm Sci Res. 2016;7(6):2274.
- Al Hanbali OA, Khan HMS, Sarfraz M, Arafat M, Ijaz S, Hameed A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharmaceutica. 2019;69(2):197–215.
- Rastogi V, Yadav P. Transdermal drug delivery system: An overview. Asian Journal of Pharmaceutics (AJP). 2012;6(3).
- Bird D, Ravindra NM. Transdermal drug delivery and patches—An overview. Med Devices Sens. 2020 Dec;3(6).
- Weiner N, Martin F, Riaz M. Liposomes as a drug delivery system. Drug Dev Ind Pharm. 1989;15(10):1523–54.
- Shashi K, Satinder K, Bharat P. A COMPLETE REVIEW ON: LIPOSOMES [Internet]. Vol. 2012, IRJP. Available from: www.irjponline.com
- SHIVALINGAM MR, BALASUBRAMANIAN A, RAMALINGAM K. FORMULATION AND EVALUATION OF TRANSDERMAL PATCHES OF PANTOPRAZOLE SODIUM. International Journal of Applied Pharmaceutics. 2021 Sep 7;287–91.
- Mo L, Lu G, Ou X, Ouyang D. Formulation and development of novel control release transdermal patches of carvedilol to improve bioavailability for the treatment of heart failure. Saudi J Biol Sci. 2022 Jan 1;29(1):266–72.
- Allena RT, Yadav HKS, Sandina S, Sarat Chandra Prasad M. Preparation and evaluation of transdermal patches of metformin hydrochloride using natural polymer for sustained release. Int J Pharm Pharm Sci. 2012;4(3):297–305.
- Cilurzo F, Gennari CGM, Minghetti P. Adhesive properties: a critical issue in transdermal patch development. Expert Opin Drug Deliv. 2012;9(1):33–45.
- Pastore MN, Kalia YN, Horstmann M, Roberts MS. Transdermal patches: history, development and pharmacology. Br J Pharmacol. 2015;172(9):2179–209.
- Bangham AD. Liposomes: the Babraham connection. Chem Phys Lipids. 1993;64(1–3):275–85.
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;975–99.
- Laouini A, Jaafar-Maalej C, Limayem-Blouza I, Sfar S, Charcosset C, Fessi H. Preparation, characterization and applications of liposomes: state of the art. Journal of colloid Science and Biotechnology. 2012;1(2):147–68.
- Jesorka A, Orwar O. Liposomes: technologies and analytical applications. Annu Rev Anal Chem. 2008;1:801–32.
- Hadian Z, Sahari MA, Moghimi HR, Barzegar M. Formulation, Characterization and Optimization of Liposomes Containing Eicosapentaenoic and Docosahexaenoic Acids; A Methodology Approach. Vol. 13, Shaheed Beheshti University of Medical Sciences and Health Services Iranian Journal of Pharmaceutical Research. 2014.
- Sangay Sherpa L, Kumar I, Chaudhary A, Lepcha B. Liposomal Drug Delivery System: Method of Preparations and Applications.
- Lichtenberg D, Barenholz Y. Liposomes: preparation, characterization, and preservation. Methods Biochem Anal. 1988;33:337–462.
- Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: Structure, composition, types, and clinical applications. Heliyon. 2022;8(5).
- Singh A, Bali A. Formulation and characterization of transdermal patches for controlled delivery of duloxetine hydrochloride. J Anal Sci Technol. 2016;7:1–13.
- Jamakandi VG, Mulla JS, Vinay BL, Shivakumar HN. Formulation, characterization, and evaluation of matrix-type transdermal patches of a model antihypertensive drug. Asian Journal of Pharmaceutics (AJP). 2009;3(1).
- Watwe RM, Bellare JR. Manufacture of liposomes: a review. Curr Sci. 1995;715–24.
- Zucker D, Marcus D, Barenholz Y, Goldblum A. Liposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical properties. Journal of controlled release. 2009;139(1):73–80.
- Gubernator J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv. 2011;8(5):565–80.
- Manish Kumar, Arpita Singh, Swarnima Pandey, Mohd. Aqil Siddiqui, Nitish Kumar. LIPOSOMES: TYPE, PREAPRATION AND EVALUATION. International Journal of Indigenous Herbs and Drugs. 2021 Feb 15;17–22.
- Kavitha K, Rajendra MM. Design and evaluation of transdermal films of lornoxicam. Int J Pharma Bio Sci. 2011;2(2):54–62.
- Barenholz Y. Relevancy of drug loading to liposomal formulation therapeutic efficacy. J Liposome Res. 2003;13(1):1–8.
- Hanumanaik M, Patil U, Kumar G, Patel SK, Singh I, Jadatkar K. Design, evaluation and recent trends in transdermal drug delivery system: a review. Int J Pharm Sci Res. 2012;3(8):2393.
- Cherukuri S, Batchu UR, Mandava K, Cherukuri V, Ganapuram KR. Formulation and evaluation of transdermal drug delivery of topiramate. Int J Pharm Investig. 2017;7(1):10.
- Sampathi S, Ajimera T, Kuchana V. PREPARATION AND EVALUATION OF LIPOSOME ENTRAPPED HYDROGEL COMPLEX SYSTEMS OF ITRACONAZOLE FOR ENHANCED TRANSDERMAL PERMEATION. Journal of Pharmaceutical & Scientific Innovation. 2014 Feb 19;3(1):25–9.
- Pastore MN, Kalia YN, Horstmann M, Roberts MS. Transdermal patches: history, development and pharmacology. Br J Pharmacol. 2015;172(9):2179–209.
- Patel RP, Patel G, Patel H, Baria A. Formulation and evaluation of transdermal patch of aceclofenac. Research Journal of Pharmaceutical Dosage Forms and Technology. 2009;1(2):108–15.
- Gaikwad AK. Transdermal drug delivery system: Formulation aspects and evaluation. Comprehensive Journal of Pharmaceutical Sciences. 2013;1(1):1–10.
- Cilurzo F, Gennari CGM, Minghetti P. Adhesive properties: a critical issue in transdermal patch development. Expert Opin Drug Deliv. 2012;9(1):33–45.
- Dhiman S, Singh TG, Rehni AK. Transdermal patches: a recent approach to new drug delivery system. Int J Pharm Pharm Sci. 2011;3(5):26–34.
- Prajapati ST, Patel CG, Patel CN. Formulation and evaluation of transdermal patch of repaglinide. Int Sch Res Notices. 2011;2011.
- Al Hanbali OA, Khan HMS, Sarfraz M, Arafat M, Ijaz S, Hameed A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharmaceutica. 2019;69(2):197–215.
- Alam MI, Alam N, Singh V, Alam MS, Ali MS, Anwer T, et al. Type, preparation and evaluation of transdermal patch: a review. World J Pharm Pharm Sci. 2013;2(4):2199–233.
- Hai NT, Kim J, Park ES, Chi SC. Formulation and biopharmaceutical evaluation of transdermal patch containing benztropine. Int J Pharm. 2008;357(1–2):55–60.
- Saroha K, Yadav B, Sharma B. Transdermal patch: A discrete dosage form. Int J Curr Pharm Res. 2011;3(3):98–108.
- Sheth NS, Mistry RB. Formulation and evaluation of transdermal patches and to study permeation enhancement effect of eugenol. J Appl Pharm Sci. 2011;(Issue):96–101.
- Mehdizadeh A, Toliate T, REZA ROUINI M, Abashzadeh S, Dorkoosh F. Design and in vitro evaluation of new drug-in-adhesive formulations of fentanyl transdermal patches. Acta Pharmaceutica. 2004;54(4):301–17.
- Aggarwal G, Dhawan S, Harikumar SL. Formulation, in vitro, and in vivo evaluation of matrix-type transdermal patches containing olanzapine. Pharm Dev Technol. 2013;18(4):916–25.
- Arora P, Mukherjee B. Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci. 2002;91(9):2076–89.
- Gupta R, Mukherjee B. Development and in vitro evaluation of diltiazem hydrochloride transdermal patches based on povidone–ethylcellulose matrices. Drug Dev Ind Pharm. 2003;29(1):1–7.
- Ren C, Fang L, Ling L, Wang Q, Liu S, Zhao L, et al. Design and in vivo evaluation of an indapamide transdermal patch. Int J Pharm. 2009;370(1–2):129–35.


 Kanika*
Kanika*


 10.5281/zenodo.10956501
10.5281/zenodo.10956501