Alzheimer's disease (AD) is a complex neurodegenerative disorder characterized by progressive cognitive decline and memory impairment. Despite extensive research, current therapeutic options remain limited, and there is no cure. Recent advancements have expanded the understanding of key molecular mechanisms underlying AD pathogenesis, paving the way for novel therapeutic approaches. This review aims to provide a comprehensive analysis of the current drug targets for AD, focusing on various receptors, enzymes, and neurotransmitters involved in disease progression. The amyloid-beta (A?) cascade, tau protein hyperphosphorylation, synaptic dysfunction, oxidative stress, neuroinflammation, and impaired neurotransmission represent major pathological hallmarks of AD. Therapeutic strategies have been developed to target these processes, including ?-secretase (BACE) and ?-secretase inhibitors for reducing amyloid deposition, tau kinase inhibitors to prevent tau hyperphosphorylation, and cholinesterase inhibitors to enhance cholinergic neurotransmission. Additionally, drugs targeting N-methyl-D-aspartate (NMDA) receptors aim to prevent excitotoxicity. Emerging therapies focus on modulating neuroinflammatory pathways, targeting microglial activation, and blocking pro-inflammatory cytokines. Receptors such as nicotinic acetylcholine receptors (nAChRs), GABA receptors, serotonin receptors, and muscarinic receptors are explored for their roles in maintaining neurotransmitter balance and neuroprotection. This review will explore these drug targets in detail, discussing both established and experimental therapeutic strategies. We will provide illustrative diagrams and tables to highlight the receptor-ligand interactions, signaling pathways, and the role of various enzymes and neurotransmitters in the progression of AD. Additionally, we will discuss the clinical efficacy and challenges of current and potential
Alzheimer’s disease, receptors, enzymes, Neurotransmitter.
3. Drug Mechanisms in Alzheimer's Disease
In Alzheimer’s, drug mechanisms are categorized into several classes based on how they target the pathophysiological features of the disease:
Cholinesterase Inhibitors:
Donepezil, Rivastigmine, Galantamine: Inhibit acetylcholinesterase, increasing acetylcholine levels to improve memory and cognition.[7]
NMDA Receptor Antagonists:
Memantine: Prevents glutamate-induced excitotoxicity by modulating NMDA receptor activity.
BACE1 and Gamma-secretase Inhibitors:
Verubecestat, Semagacestat: Inhibit the enzymes responsible for amyloid-beta plaque formation (though clinical trials have seen limited success).
Tau Phosphorylation Inhibitors:
Tideglusib:
Inhibits GSK-3? to reduce hyperphosphorylation of tau proteins and prevent neurofibrillary tangle formation.
- Tau Phosphorylation Pathway and Neurofibrillary Tangle (NFT) Formation
Tau is a protein responsible for stabilizing microtubules, which are crucial for the structural integrity and function of neurons. In Alzheimer's disease, abnormal hyperphosphorylation of tau leads to the formation of neurofibrillary tangles (NFTs), one of the hallmarks of the disease.[8]
Mechanism of Tau Phosphorylation:
- GSK-3? (Glycogen Synthase Kinase-3?) and CDK5 (Cyclin-dependent kinase 5) are key enzymes responsible for phosphorylating tau.
- Under normal conditions, tau phosphorylation is tightly regulated. However, in AD, these enzymes are overactive, causing excessive phosphorylation.
- Hyperphosphorylated tau detaches from microtubules and aggregates into paired helical filaments (PHFs), eventually leading to NFT formation.[9]
- Impact of NFTs:
- NFTs disrupt intracellular transport by disassembling microtubules, leading to impaired synaptic function and cell death.
- The accumulation of NFTs is associated with cognitive decline and correlates with the severity of dementia in AD patients.[10]
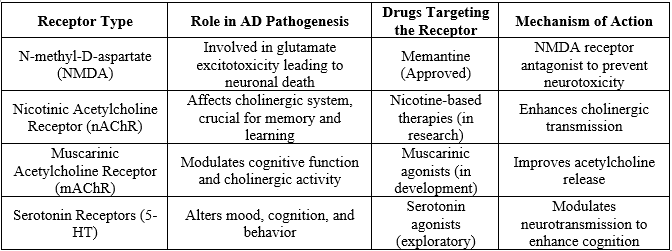
- Neurotransmitter Receptor Subtypes in Alzheimer's Disease
The dysregulation of neurotransmitter systems is a key contributor to the cognitive deficits seen in AD. The most affected neurotransmitter systems include acetylcholine, glutamate, and serotonin. Let’s explore their receptor subtypes.
Acetylcholine Receptors:
Muscarinic Receptors (M1, M2):
These are G-protein-coupled receptors involved in various cognitive functions, including memory and learning.
- M1 receptors are mainly associated with enhancing cognitive function.
- M2 receptors regulate the release of acetylcholine and are linked to controlling heart rate and muscle contraction.[11]
- Drugs in development target muscarinic receptors to restore cognitive abilities in AD.
Nicotinic Receptors (?4?2, ?7):
These receptors play a critical role in synaptic plasticity and memory formation.
- ?7 nicotinic receptors are crucial for attention and working memory.
- Nicotine-based therapies are being explored to activate these receptors and improve cholinergic transmission in AD patients.[12]
Glutamate Receptors:
NMDA Receptors:
- NMDA receptors are critical for synaptic plasticity and memory formation. In AD, overactivation of these receptors by excess glutamate leads to excitotoxicity, causing neuronal damage.
- Memantine, an NMDA receptor antagonist, is used to prevent this excitotoxicity.[13]
AMPA Receptors:
- AMPA receptors mediate fast excitatory transmission in the brain and are crucial for long-term potentiation (LTP), a process involved in learning and memory.
- AMPA receptor modulators are being investigated as potential therapies to enhance synaptic strength in AD.[14]
Serotonin Receptors:
- 5-HT1A and 5-HT2A receptors are key in modulating mood, behavior, and cognition.
- 5-HT1A agonists are being explored to improve cognitive function and reduce neuroinflammation in AD.
- Selective serotonin reuptake inhibitors (SSRIs), which increase serotonin levels, are used to treat mood disorders in AD patients.[15]
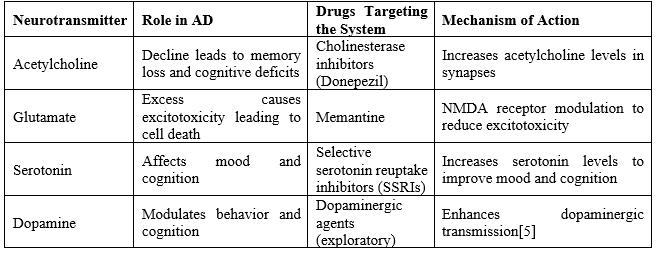
3. Drug Mechanisms in Alzheimer's Disease
Different drug classes target the biochemical pathways involved in AD, focusing on reducing cognitive decline and preventing further neuronal damage. Here are the major drug classes
Cholinesterase Inhibitors:
- Donepezil, Rivastigmine, and Galantamine are cholinesterase inhibitors that prevent the breakdown of acetylcholine by inhibiting acetylcholinesterase (AChE). This increases acetylcholine availability in synapses, improving memory and cognitive function.[16]
- These drugs are primarily used in early to moderate stages of AD to provide symptomatic relief.
NMDA Receptor Antagonists:
Memantine is the only approved NMDA receptor antagonist for moderate to severe AD. It works by blocking excessive NMDA receptor activation caused by high levels of glutamate, thereby preventing excitotoxicity and protecting neurons from damage. [17]
BACE1 and Gamma-secretase Inhibitors:
- BACE1 (beta-secretase) and gamma-secretase are enzymes involved in the production of amyloid-beta (A?) plaques.
- BACE1 inhibitors (such as verubecestat and lanabecestat) aim to block the first step in amyloid-beta production. However, clinical trials have faced challenges, as these inhibitors have shown limited efficacy and significant side effects.
- Gamma-secretase inhibitors were also explored but similarly failed in clinical trials due to adverse effects on other important biological processes.[18]
Tau Phosphorylation Inhibitors:
Tideglusib, a GSK-3? inhibitor, aims to reduce the hyperphosphorylation of tau proteins, preventing the formation of NFTs. This drug showed some promise in early trials, though further research is needed to establish its long-term efficacy.
- Tau Phosphorylation Pathway: Detailed Mechanism
Tau Protein:
- Normally, tau stabilizes microtubules in neurons, aiding in intracellular transport, which is vital for neuronal health.
- Hyperphosphorylated tau: In Alzheimer's, tau becomes abnormally phosphorylated by enzymes such as GSK-3? and CDK5. This results in tau dissociating from microtubules and accumulating in the cytoplasm, forming insoluble aggregates known as paired helical filaments (PHFs).
GSK-3? and CDK5:
- GSK-3? (Glycogen Synthase Kinase-3?) is one of the main kinases responsible for tau hyperphosphorylation. Overactivation of GSK-3? is a significant contributor to tau aggregation in AD.
- CDK5 (Cyclin-dependent kinase 5) plays a role in abnormal phosphorylation of tau in neurodegenerative conditions. [19]
Neurofibrillary Tangles (NFTs):
- Tau tangles interfere with neuron function by disrupting the microtubule network. NFTs accumulate inside neurons, leading to synaptic dysfunction and neuronal death. They spread through the brain in a predictable pattern, starting in the entorhinal cortex and hippocampus (areas involved in memory) before spreading to the neocortex.
Therapeutic Targeting:
- Tideglusib, a GSK-3? inhibitor, aims to reduce tau hyperphosphorylation. By inhibiting GSK-3?, tideglusib potentially prevents tau from forming neurofibrillary tangles. Although promising, clinical trials have produced mixed results, necessitating further research.[20]
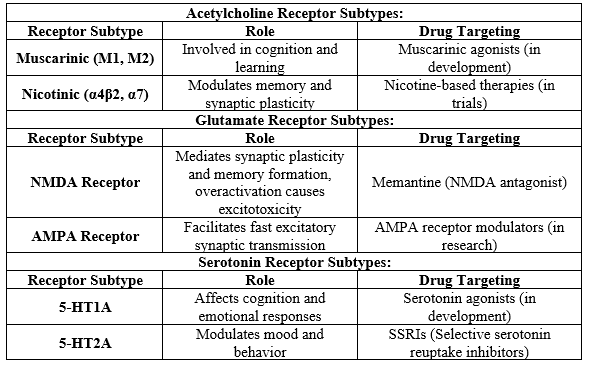
2. Neurotransmitter Receptor Subtypes: Detailed Insights
Acetylcholine Receptors in Alzheimer's:
The cholinergic hypothesis proposes that a loss of cholinergic neurons in the basal forebrain contributes to the cognitive decline observed in Alzheimer's disease.
Muscarinic Receptors:
- M1 receptors are involved in cognitive function, and their stimulation can improve cognitive processes. Research is focusing on selective muscarinic agonists to improve memory in AD.
- M2 receptors are inhibitory autoreceptors that modulate acetylcholine release. Targeting these receptors could provide a balanced approach to enhancing cholinergic activity without causing overstimulation.[21]
Nicotinic Receptors:
- ?4?2 and ?7 nicotinic acetylcholine receptors (nAChRs) are implicated in attention, memory, and synaptic plasticity. These receptors are found to be significantly reduced in AD brains.
- Therapies like nicotine patches or selective agonists targeting ?7 receptors are under investigation to improve cognitive function and reduce neuroinflammation.
Glutamate Receptors in Alzheimer's:
Glutamate excitotoxicity:
In AD, excessive glutamate overstimulates NMDA receptors, leading to calcium influx, which triggers apoptosis (programmed cell death).
- Memantine acts as an NMDA receptor antagonist, blocking excessive glutamate stimulation without affecting normal synaptic activity. This drug helps prevent excitotoxicity while preserving cognitive function.[22]
AMPA Receptors:
AMPA receptors are also involved in synaptic transmission. Dysregulation of these receptors in AD can impair synaptic plasticity and learning. AMPA modulators are in experimental stages to enhance synaptic function and memory.
Serotonin Receptors in Alzheimer's:
These receptors modulate mood and cognitive functions. Agonists of 5-HT1A may reduce neuroinflammation and improve cognition.
These receptors play a role in mood regulation and are targeted by antidepressants, such as SSRIs (Selective Serotonin Reuptake Inhibitors), which are commonly prescribed to AD patients for managing depression and anxiety.[23]
3. Drug Mechanisms: Expanded Details
Cholinesterase Inhibitors:
Mechanism:
Cholinesterase inhibitors work by blocking the breakdown of acetylcholine by acetylcholinesterase (AChE) in the synaptic cleft, thereby increasing the availability of acetylcholine for neurotransmission.
Donepezil:
Widely prescribed for all stages of AD, donepezil is a reversible inhibitor of AChE and helps improve cognitive symptoms.
Rivastigmine:
A dual inhibitor of AChE and butyrylcholinesterase (BuChE), rivastigmine is used for mild to moderate AD and is also available as a transdermal patch.
Galantamine:
In addition to AChE inhibition, galantamine acts as an allosteric modulator of nicotinic receptors, enhancing cholinergic transmission.[24]
NMDA Receptor Antagonists:
Memantine:
Memantine selectively blocks pathological overactivation of NMDA receptors without affecting normal receptor activity, thereby reducing glutamate-induced excitotoxicity.
- Clinical benefits include improved memory, attention, and overall cognitive function in patients with moderate to severe AD.[25]
BACE1 and Gamma-secretase Inhibitors:
BACE1 Inhibitors:
These drugs aim to prevent the formation of amyloid-beta (A?) by inhibiting the BACE1 enzyme that initiates the cleavage of amyloid precursor protein (APP).
- Verubecestat and Lanabecestat were promising BACE1 inhibitors, but trials were halted due to side effects and lack of efficacy in cognitive outcomes.[25]
Gamma-secretase Inhibitors:
These drugs target the enzyme that produces amyloid-beta after BACE1 cleavage. However, gamma-secretase also processes other important proteins, leading to adverse effects, such as gastrointestinal problems and skin disorders, resulting in discontinuation of many trials.
Tau Phosphorylation Inhibitors:
Tideglusib:
By inhibiting GSK-3?, this drug prevents the abnormal hyperphosphorylation of tau. Although it showed potential in early trials, further research is needed to optimize its efficacy and minimize side effects.[26]
1. Tau Phosphorylation Pathway: Clarification and Future Directions
Tau pathology is a key driver of neurodegeneration in Alzheimer's disease. The following additional points and emerging therapies focus on new strategies to modulate tau:
- Abnormal Hyperphosphorylation: In Alzheimer's disease, tau is phosphorylated at numerous sites, with some specific sites being especially crucial for tau's toxicity. Hyperphosphorylated tau aggregates into paired helical filaments (PHFs), which later coalesce into neurofibrillary tangles (NFTs).
- Spreading of Tau Pathology: Emerging research suggests that tau pathology spreads in a prion-like fashion, moving from one neuron to another, potentially accelerating disease progression. This makes tau-targeted therapies even more critical in halting or slowing disease spread.[27]
New Therapeutic Directions for Tau:
Anti-tau Antibodies:
One of the promising approaches involves using monoclonal antibodies to target pathological tau and prevent its spread. Clinical trials, such as semorinemab (anti-tau antibody), are ongoing to test their efficacy in reducing tau aggregation.
Tau Vaccines:
These vaccines aim to stimulate the immune system to clear pathological tau from the brain. Early clinical trials have shown that tau-targeting vaccines may reduce tau burden in animal models, and human trials are underway.[28]
2. Neurotransmitter Receptor Subtypes in Alzheimer's: Clarification and Potential Developments
Acetylcholine Modulation
The Decline of Cholinergic Neurons:
In AD, basal forebrain cholinergic neurons are severely affected, resulting in reduced levels of acetylcholine. The cholinergic hypothesis postulates that this loss leads to the hallmark symptoms of memory loss and cognitive decline.
Muscarinic Receptor Agonists:
AF102B and Xanomeline:
These are selective M1 muscarinic receptor agonists that aim to restore cognitive function by enhancing cholinergic transmission. Early trials with xanomeline showed promise in improving cognitive symptoms in AD patients, although side effects like nausea and vomiting have been a challenge.[29]
Glutamate Modulation:
Excitotoxicity:
The overactivation of NMDA receptors by glutamate leads to an influx of calcium into neurons, triggering cell death pathways. Preventing this overactivation without disrupting normal glutamate signaling is key to preserving cognitive function.
Potential NMDA Modulators:
SAGE-718:
A drug that modulates NMDA receptor activity, currently being studied for its potential to improve cognitive function in early Alzheimer's. It aims to enhance NMDA function selectively without causing excitotoxicity.[30]
AMPA Receptor Modulators:
Enhancing AMPA receptor activity may boost synaptic transmission and cognitive function. Drugs like CX-516 (AMPAkine) are under investigation for their ability to improve cognitive outcomes by facilitating synaptic plasticity.
Serotonin Modulation
5-HT1A Agonists: These drugs are being studied for their ability to modulate cognitive processes and mood. Serotonin reuptake inhibitors are already commonly used to manage depression and anxiety in AD patients, but selective serotonin modulators might enhance cognitive benefits.
3. Drug Mechanisms in Alzheimer’s Disease: Additional Clarifications and Future Prospects
Cholinesterase Inhibitors:
Future Developments:
Researchers are exploring next-generation cholinesterase inhibitors that are more selective or have fewer side effects than current drugs like donepezil. New delivery methods, such as transdermal patches, are also being studied to reduce gastrointestinal side effects commonly seen with oral medications.[31]
NMDA Receptor Antagonists:
Memantine's Success:
- Memantine is often used in combination with cholinesterase inhibitors to provide a synergistic effect in treating moderate to severe AD. However, it only provides symptomatic relief rather than addressing the underlying pathology.
- Next-Generation NMDA Antagonists: Nitromemantine is a derivative of memantine, developed to improve the specificity of NMDA receptor blocking and reduce potential side effects. It’s still in the experimental phase but shows promise. [32]
BACE1 Inhibitors:
Why Clinical Trials Struggled:
The failure of drugs like verubecestat in BACE1 inhibition trials stemmed from their lack of efficacy and high levels of adverse effects, including worsening cognition. This suggests that targeting amyloid-beta production alone may not be sufficient to halt disease progression.
Rethinking Amyloid Pathway Targeting:
Research is now focusing on more selective BACE1 inhibitors that can modulate amyloid-beta production without completely shutting down the pathway, thus minimizing off-target effects.[33]
Gamma-secretase Inhibitors:
Gamma-secretase Modulators (GSMs):
These modulate gamma-secretase activity without fully inhibiting it, aiming to reduce amyloid-beta production while avoiding side effects. GSMs are seen as a more promising approach compared to earlier gamma-secretase inhibitors, which had systemic toxicity.
Tau-targeting Therapies:
Ongoing Trials:
In addition to tideglusib, several other tau-targeting drugs are in trials:
Zagotenemab:
Another anti-tau monoclonal antibody that targets extracellular tau to prevent its spread between neurons.
TRx0237 (LMTX):
This drug is thought to disassemble tau tangles and prevent their aggregation, potentially reducing tau pathology.
Emerging Concepts in Alzheimer's Drug Development
Targeting Neuroinflammation:
Inflammation in AD:
- Chronic neuroinflammation is a significant factor in Alzheimer’s disease progression. Drugs targeting microglial activation (the brain’s immune cells) are being explored to reduce inflammation.
- Neflamapimod, a p38 MAPK inhibitor, aims to reduce inflammatory signaling in the brain. Clinical trials have shown that reducing inflammation may slow cognitive decline.[34]
Restoring Synaptic Plasticity:
Synaptic dysfunction occurs early in Alzheimer's disease, leading to cognitive deficits. Therapies aimed at enhancing synaptic plasticity, such as BDNF (brain-derived neurotrophic factor) mimetics, are being developed to restore neuronal communication and memory function.
LM22A-4, a small molecule that mimics BDNF signaling, is currently in preclinical testing for its potential to restore synaptic plasticity.[35]
Gene Therapy and CRISPR:
Gene-editing technologies like CRISPR-Cas9 are being explored to target genetic mutations (e.g., in APOE4) that increase Alzheimer’s disease risk. While still experimental, gene therapy holds long-term potential for halting or even reversing neurodegeneration.
1. Neuroinflammation in Alzheimer’s Disease
Chronic Neuroinflammation is increasingly recognized as a major contributor to Alzheimer's pathology. In the brain, inflammation is primarily mediated by microglia, the brain’s resident immune cells. While microglia help clear amyloid-beta deposits in early AD, chronic activation leads to the release of pro-inflammatory cytokines, contributing to neurodegeneration.[36]
Microglial Activation and Therapeutic Approaches
Microglia play a dual role: protective early on by clearing amyloid-beta but harmful as the disease progresses, contributing to synaptic loss and tau propagation.
Therapies Targeting Microglia:
Neflamapimod:
A p38 MAPK inhibitor that aims to reduce neuroinflammatory signaling in microglia. By inhibiting this pathway, neflamapimod could reduce synaptic dysfunction and improve cognitive outcomes. Clinical trials have shown it reduces inflammation and may slow memory loss.[37]
AL002:
This monoclonal antibody targets the TREM2 receptor on microglia, which plays a crucial role in the microglial response to amyloid plaques. Early studies suggest that enhancing TREM2 activity could promote the clearance of amyloid-beta and reduce inflammation.
Role of Astrocytes in Inflammation:
Astrocytes also play a role in Alzheimer's neuroinflammation. They become reactive and produce inflammatory molecules, which exacerbate synaptic damage. Therapies targeting astrocytes are still in the exploratory phase but could offer additional routes for managing inflammation.[38]
- Restoring Synaptic Plasticity:
A Focus on BDNF and Neurotrophic Factors
Synaptic plasticity—the ability of synapses to strengthen or weaken over time—is crucial for learning and memory. In AD, synapses are damaged early, long before significant neuron loss. Enhancing synaptic plasticity could improve cognitive function, even in the presence of amyloid-beta and tau pathology.
Brain-Derived Neurotrophic Factor (BDNF) and Mimetics
BDNF is a neurotrophic factor that supports the survival of existing neurons and encourages the growth and differentiation of new neurons and synapses. In Alzheimer's, BDNF levels are reduced, which contributes to cognitive decline.
BDNF-Based Therapeutics:
LM22A-4:
A small molecule that mimics the action of BDNF by selectively activating its receptor, TrkB. By stimulating TrkB, LM22A-4 promotes synaptic plasticity and may reverse cognitive deficits in AD. Early studies in animal models show improved memory and synaptic function.
AAV-BDNF Gene Therapy:
This therapy delivers BDNF directly to affected brain regions using an adeno-associated virus (AAV) vector. Early trials in animal models have shown that increasing BDNF expression improves memory and reduces neuron loss, providing hope for its potential in human clinical trials.[39]
3. Gene Therapy and CRISPR in Alzheimer’s Disease
Gene therapy is emerging as a potential solution for targeting specific genetic risk factors associated with Alzheimer’s disease, particularly for individuals carrying high-risk mutations like APOE4. [40]
APOE4 and Gene Editing
APOE4 is the most significant genetic risk factor for Alzheimer's, increasing the risk of developing the disease by 3- to 15-fold compared to those carrying the APOE3 variant.
CRISPR-based Gene Therapy:
CRISPR-Cas9 gene-editing technology is being explored to modify the APOE4 gene and convert it into the less harmful APOE2 or APOE3 forms. Early research in animal models shows that CRISPR can successfully reduce APOE4 production, potentially lowering the risk of amyloid plaque formation.
AAV-APOE2 Therapy:
In this therapy, a virus is used to introduce the protective APOE2 gene into the brain. This approach is being studied as a potential treatment for individuals with the APOE4 gene to reduce their risk of developing Alzheimer's.[41]
4. Drug Trials and Future Prospects
Several clinical trials are underway, exploring new compounds and mechanisms that aim to slow or halt Alzheimer's progression. Here's an update on some of the most advanced and promising drug candidates:
Aducanumab and Anti-Amyloid Antibodies
Aducanumab (approved in 2021) was the first FDA-approved drug to target amyloid-beta directly. However, its approval was controversial due to mixed trial results. While it effectively clears amyloid plaques, its impact on cognitive decline remains unclear.
Lecanemab:
Another anti-amyloid antibody currently in phase III trials. Preliminary results suggest that it reduces amyloid plaques and shows more promise than aducanumab in slowing cognitive decline. [42]
Anti-Tau Therapies
Semorinemab:
A tau-targeting monoclonal antibody designed to block extracellular tau and prevent it from spreading between neurons. While its first trials were not successful, ongoing studies are adjusting dosages and targeting different disease stages.
Zagotenemab:
Another anti-tau antibody, zagotenemab is designed to clear extracellular tau aggregates and prevent their propagation. Early clinical trials are underway to evaluate its long-term benefits on cognitive function.
Next-Generation BACE1 Inhibitors
Following the failure of first-generation BACE1 inhibitors, new approaches aim to develop more selective compounds with fewer side effects. These include:
LY3202626:
A selective BACE1 inhibitor that showed some efficacy in early trials, but further research is needed to determine its long-term effects.[43]
5. Innovative Approaches: Combining Therapies
As Alzheimer's is a multifaceted disease, there’s increasing interest in combination therapies that target multiple pathways. Some ongoing studies focus on combining cholinesterase inhibitors, NMDA antagonists, and anti-amyloid or anti-tau therapies to tackle the disease from different angles.
Multi-Target Drugs
Anavex 2-73 (blarcamesine):
A sigma-1 receptor agonist and muscarinic receptor modulator that shows neuroprotective effects. Anavex 2-73 is being studied for its ability to reduce amyloid-beta toxicity, modulate synaptic plasticity, and prevent tau hyperphosphorylation. Phase II trials are promising, and phase III studies are underway.
Tricaprilin:
A ketogenic compound designed to improve mitochondrial function and energy production in neurons. Early clinical studies have shown that enhancing metabolic function may improve cognition in patients with mild to moderate AD.
6. The Future of Alzheimer's Disease Research
The future of Alzheimer's disease treatment lies in early intervention and personalized medicine. Some trends and future directions include:
- Biomarkers for Early Diagnosis:
Advances in fluid biomarkers (e.g., blood or cerebrospinal fluid tests for amyloid-beta and tau) and imaging techniques (e.g., PET scans) are helping to identify Alzheimer's at the preclinical stage. Early diagnosis will allow for earlier treatment, improving the chances of success.
Tailoring therapies based on genetic risk factors, such as APOE status or the presence of specific tau mutations, could lead to more effective treatments. Precision medicine in AD could also involve genetic profiling and adjusting drug combinations to suit individual needs.
Along with pharmacological treatments, research is increasingly focusing on lifestyle interventions, such as diet, exercise, and cognitive training, which have been shown to delay the onset or progression of AD in some cases. [44]
1. Amyloid Beta (A?) Plaques
Overview:
Amyloid beta plaques are aggregates of misfolded amyloid beta peptides, which are believed to contribute to neurodegeneration in Alzheimer's disease.
Drug Targets:
Amyloid-beta Monoclonal Antibodies: Aim to reduce amyloid plaque burden.
Examples:
Aducanumab, Lecanemab, Donanemab.


 C. Rejitha*
C. Rejitha*



 10.5281/zenodo.13850740
10.5281/zenodo.13850740