Abstract
Amphetamine (AMPH) and its derivatives, exhibiting diverse structures and psychoactive effects, share similarities with the botanical diversity of orchids. This review delves into the multifaceted mechanisms underlying their primary biological impact: the elevation of extracellular catecholamines and serotonin levels. We explore these mechanisms independently of classical transmitter release pathways, providing historical context and future research directions. While traditionally referred to in plural form, we cautiously define "the amphetamines" to encompass compounds sharing an ?-methyl-phenethyl-amine motif. Focusing primarily on AMPH and methamphetamine (METH), exemplary catecholamine releasers, we discuss subtle distinctions between these compounds and their differential effects. Furthermore, we examine the historical utilization of natural plant-derived amphetamines, tracing their origins from Ephedra species to contemporary clinical applications. Additionally, we explore the role of trace amines in affective modulation and their potential as endogenous amphetamines. Lastly, we discuss the evolution and impact of synthetic amphetamines, from their inception to contemporary epidemics and clinical uses. Through comprehensive exploration of amphetamine actions on synaptic vesicles and plasma membrane transporters, we unravel their complex pharmacological effects and therapeutic implications
Keywords
Amphetamine, catecholamines, serotonin, neurotransmission, synaptic vesicles, plasma membrane transporters, mechanisms of action, historical utilization, synthetic amphetamines, pharmacological effects
Introduction
Amphetamine (AMPH) and its various derivatives exhibit a wide range of structural diversity and psychoactive effects, akin to the diversity found in orchids within the plant kingdom [1, 2]. This review explores the multiple mechanisms underlying their primary biological effect: the elevation of extracellular levels of catecholamines and serotonin. This effect occurs independently of the classical mechanisms involved in transmitter release via secretory vesicle fusion [3, 4]. We discuss current concepts within a historical context to provide a framework for future research on this class of drugs, which is both rewarding and addictive, popular yet despised, and capable of both beneficial and destructive effects. Although Alexander Shulgin (1978) argued for the singular use of the term "amphetamine" to denote a specific chemical, linguistic conventions persist in referring to these compounds in the plural form due to the inclusion of the term in the generic names of various compounds sharing the AMPH structure[5, 6]. For the purpose of this review, we define "the amphetamines" cautiously to encompass compounds sharing an ?-methyl-phenethyl-amine motif, as suggested by the generic name of the archetypal compound. Biel and Bopp (1978) outlined the definitive structural features of AMPH, including an unsubstituted phenyl ring, a two-carbon side chain between the phenyl ring and nitrogen, an ?-methyl group, and a primary amino group [7, 8, 9]. The focus of this article is primarily on AMPH and methamphetamine (METH), both exemplary catecholamine releasers with minimal affinity for neuronal receptors, simplifying our review. Although METH deviates from the fourth rule by possessing a secondary amine, it remains one of the most studied compounds in this class [10]. While we do not delve into comparisons of how chemical substitutions alter the efficacy of AMPH-related drugs across various mechanisms, we occasionally reference chain-substituted and ring-substituted amphetamines, as well as non-?-methylated phenethylamines, for comparison. The question often arises regarding the differential effects of AMPH and METH. Studies indicate no significant differences in terms of dopamine release in the striatum, elimination rates, or other pharmacokinetic properties [11, 12]. Human discrimination studies also fail to distinguish between equal doses of the two drugs. However, subtle distinctions exist, such as slightly greater dopamine release by AMPH in the prefrontal cortex, leading to nuanced differences in effects on working memory and behavioral tolerance [13, 14]. AMPH also tends to induce slightly more locomotor activity in rodents than METH, possibly due to indirect effects [15, 16]. Claims suggesting that METH is more addictive, preferred by drug addicts, more potent as a psychostimulant, or exhibits diminished peripheral activity appear unsubstantiated [17, 18]. Traditionally, studies on mechanisms of action focus on AMPH, while studies on neurodegeneration primarily involve METH [19, 20]. This discrepancy may be attributed to the greater availability of METH on the illicit market due to its simpler synthesis methods, which involve either a one-step reduction of ephedrine or pseudoephedrine, or a condensation of phenylacetone and methylamine. The former method yields only the more active S(+)-enantiomer due to the stereochemical purity of ephedrine, a natural product [21, 22, 23].
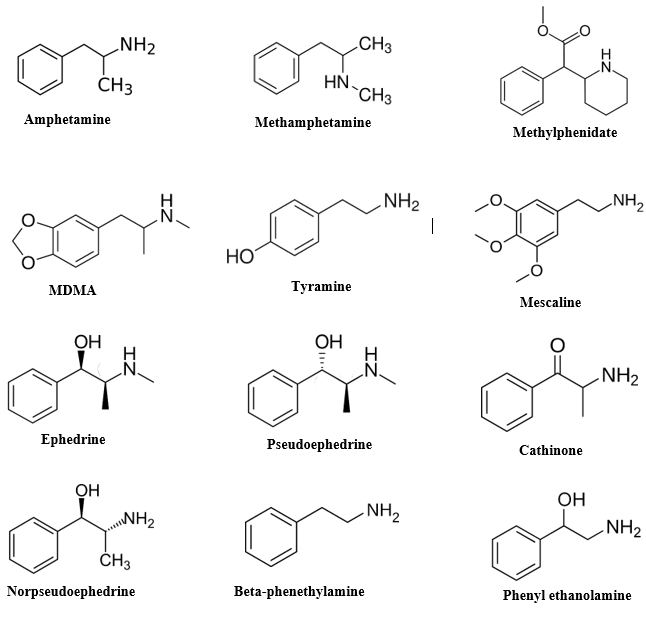
Historical Utilization of Natural Plant-Derived Amphetamines
Similar to nicotine, cocaine, opiates, marijuana, and alcohol, amphetamines have been utilized in their natural plant forms for thousands of years [24, 25, 26]. Ephedra species and the Catha edulis tree, commonly known as khat, have historically been the principal sources of these plant-based stimulants [27, 28]. Ephedra sinica, referred to as ephedra or Ma huang in China, has been unearthed from ancient gravesites in the Middle East and Indian Vedic temples, with controversial associations to soma, a sacred substance in Vedic rituals [29]. Ephedrine, its primary active compound, was identified in the late 19th century. In Western regions, a stimulant tea brewed from a different Ephedra species, Mormon Tea (E. nevadensis), gained popularity, particularly as it was not restricted by the Church of Jesus Christ of Latter-day Saints [30, 31]. However, recent studies suggest that New World Ephedra lacks the alkaloids found in the original ephedrine-containing species [32, 33]. Ephedrine became widely used as an over-the-counter appetite suppressant and performance enhancer, although its dangers were underscored by incidents such as the death of Baltimore Orioles pitcher Steve Bechler in 2003 [34, 35]. Its usage as a dietary supplement was subsequently banned by the FDA in 2004, primarily due to its role as a precursor in the illicit production of methamphetamine [36, 37]. Khat, derived from the leaves of Catha edulis, contains the amphetamines cathinone and cathine [38, 39]. Its use dates back to the 11th century and is prevalent in regions such as Yemen, Kenya, and Somalia [40]. Khat consumption is associated with sociability, euphoria, and appetite suppression [41]. Despite its widespread use, khat is habit-forming and can lead to paranoia and other psychological disturbances [42]. However, withdrawal symptoms are reported to be relatively mild. Khat chewing is deeply embedded in social customs, particularly in Yemen, where dedicated rooms are often set aside for communal khat sessions [43]. While other plant species contain natural amphetamines, including certain Acacia species and Egyptian jasmine, they are not commonly used for administration of these drugs [44, 45]. However, there are plants containing components similar to amphetamines, such as synephrine found in citrus plants and arecoline in the betel nut palm [46, 47]. Mescaline, a phenethylamine analog found in the peyote cactus, has a distinct mechanism of action compared to amphetamines, but has inspired the synthesis of numerous amphetamine derivatives. It has been historically used in Native American rituals and is protected by law for religious purposes [48, 49, 50].
Role of Trace Amines in Affective Modulation and Potential Endogenous Amphetamine Action
It has been long established that decarboxylated metabolites of aromatic amino acids, such as ?-phenethylamine from phenylalanine, phenylethanolamine, tyramine from tyrosine, and tryptamine from tryptophan, are synthesized in both the peripheral nervous system and the brain [51, 52, 53]. These compounds have been implicated in modulating affective behaviors such as excitement and alertness. Interestingly, decreased levels of these "trace amines" have been observed in the urine of depressed patients, while their levels are elevated by marijuana [54].Although trace amines share some mechanisms of action with amphetamines and may potentially act as endogenous amphetamines, they are not stored in substantial quantities in either the central nervous system or the periphery. Exogenous administration of high levels of ?-phenethylamine or its accumulation due to monoamine oxidase (MAO) inhibition can induce psychostimulant responses similar to amphetamines [55, 56]. Like amphetamines, ?-phenethylamine releases dopamine in a manner dependent on the presence of an intact dopamine transporter (DAT) [57]. However, certain behavioral responses to ?-phenethylamine appear to be independent of DAT. The neurotransmitter status of ?-phenethylamine remains uncertain. When present in high concentrations, it is likely to be transported into synaptic vesicles by the vesicular monoamine transporter [58, 59]. However, some studies suggest that ?-phenethylamine may also be released by diffusion across membranes, rather than through reverse transport via catecholamine transporters, and may exhibit insensitivity to reserpine, an inhibitor of vesicular catecholamine uptake [60, 61]. In contrast to amphetamines, trace amines lack the ?-methyl group that inhibits MAO, making them rapidly metabolized in the brain [62]. It is unclear whether local concentrations of ?-phenethylamine in the brain are sufficient to elicit endogenous amphetamine-like effects or activate receptors significantly, although some trace amine compounds show high affinity for recently identified trace amine receptors [63, 64, 65].
The Evolution and Impact of Synthetic Amphetamine: From Invention to Epidemics and Clinical Applications
Synthetic amphetamine (AMPH) was first synthesized in 1887 by Lazar Edeleanu, a Romanian chemist who described its production in his doctoral dissertation under A.W. Hofmann at the University of Berlin [66, 67]. Edeleanu later gained recognition for inventing the method to distill petroleum using sulfur dioxide, which imparted the distinctive odor to gasoline distilleries [68, 69]. AMPH derives its generic name from a contraction of "?-methyl-phenethyl-amine." The rich history of this compound is evident from the 1989 Merck Index, which listed 17 trade names, excluding well-known brands like Adderall, Benzedrine, and Dexedrine, along with numerous nicknames used by drug abusers. Over time, chemists have developed an extensive array of synthetic AMPH derivatives [70, 71]. The concept of sympathomimetic amines, introduced by Barger and Dale, spurred research into various catecholamine-like derivatives for their ability to raise blood pressure and relieve nasal and bronchial congestion [72, 73]. Independently, Gordon Alles resynthesized AMPH in 1927 as part of efforts to develop synthetic sympathomimetics [74]. Walter Hartung and James Munch identified AMPH as a potent sympathomimetic and particularly effective when administered orally. Commercially introduced in 1932 as Benzedrine by the pharmaceutical firm Smith, Kline and French, AMPH gained popularity rapidly. Its first clinical use, for narcolepsy, was reported by Myron Prinzmetal in 1935 [75, 76]. By 1936, Benzedrine tablets became available without prescription, leading to widespread usage, notably among students, artists, musicians, and truck drivers. Initially promoted for over 30 uses, including schizophrenia treatment and seasickness relief, by 1946, AMPH's annual pharmaceutical production reached 10 billion tablets, with a significant portion diverted to the black market [77, 78]. In 1971, legal quotas on AMPH production were imposed by the United States Justice Department. Despite this, AMPH, methamphetamine, and methylphenidate remain widely prescribed for weight control, narcolepsy, and attention deficit disorder [79, 80]. Military use of AMPH for promoting alertness dates back to the Spanish Civil War, with ongoing usage, particularly among fighter pilots [81]. Reports of AMPH abuse and psychosis emerged soon after its introduction, with concerns over addiction becoming more prominent in the mid-1960s. Similar to cocaine, AMPH use tends to occur in epidemic waves, as seen in Japan and Sweden during the mid-20th century [82, 83]. Currently, methamphetamine is the most prevalent illicitly manufactured controlled substance in the United States, with widespread local epidemics reported. MDMA, originally developed for clinical use, remains popular illicitly, despite being assigned Schedule I status in 1985 [84, 85].
Efforts to synthesize AMPH derivatives have led to the exploration of various compounds for clinical applications, including appetite suppression and treatment of Parkinson's Disease [86].
Exploring Multifaceted Sites of Action: A Comprehensive Overview
Early Interplay Between Amphetamine Action and Adrenal Medulla Secretion
Early scientific exploration into the action of amphetamines (AMPH) is intricately tied to the study of the adrenal medulla and its secretion of catecholamines. Catechol, originating from catechu, a plant extract, was traditionally used for dyeing fabric and medicinal purposes [87, 88, 89]. George Oliver, a British physician, observed increased blood pressure upon injecting adrenal gland extract into his son [90, 91]. By 1894, Oliver and Edward Albert Schafer confirmed this effect in dogs [92]. Epinephrine, the active compound, was identified independently by three laboratories in 1897. Its structure was elucidated by Ernst Joseph Friedman in 1906. These discoveries laid the groundwork for the hypothesis of secretory transmission, suggesting that nerves communicate through chemical release [93, 94, 95].
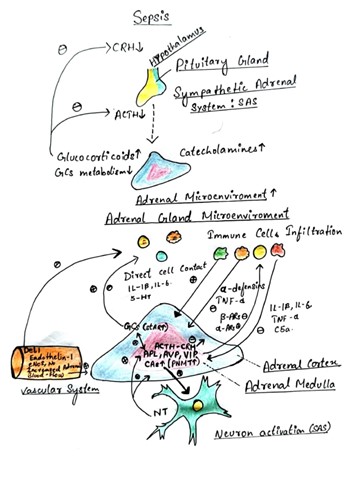
Fig no 2. Mechanism of Action of Amphetamine in Adrenal medulla
Thomas Renton Elliott proposed this idea in 1904 after confirming epinephrine's effects. He also suggested nerve accumulation of epinephrine, though this was demonstrated much later. Pharmacological exploration began with George Barger and Henry H. Dale in 1910, who investigated compounds mimicking epinephrine's effects [96, 97, 98]. They coined the term "sympathomimetic" to describe such compounds. Later research by J.H. Burn and colleagues distinguished between directly and indirectly acting sympathomimetics [99]. Cocaine, derived from Erythoxylon coca, provided insights into physiological mechanisms, particularly as a monoamine uptake transporter inhibitor. Its effects, including its association with Coca-Cola and initial medical enthusiasm, were noted [101, 102]. Alfred Fröhlich and Otto Loewi's work in 1910 on cocaine's interaction with epinephrine added to this understanding [103, 104]. Reserpine, derived from Rauwolfia serpentina, was pivotal in unraveling the actions of sympathomimetics. Its introduction to the West led to significant therapeutic applications, albeit with notable side effects [105, 106]. Arvid Carlsson and others in 1957 showed reserpine's interaction with tyramine, shedding light on sympathomimetic actions [107, 108]. Burn and Rand's 1958 study clarified amphetamines' mechanism by showing their ability to release catecholamines. This explained differences in their actions compared to uptake blockers like cocaine [109, 110]. Later research confirmed amphetamines' role in central dopamine release, emphasizing release over reuptake blockade as the primary mechanism.
Overall, these early investigations laid the foundation for understanding amphetamine action, with subsequent studies refining our knowledge of its physiological effects [111].
Exploring the Role of Plasma Membrane Uptake Transporters and Monoamine Secretory/Synaptic Vesicles in Amphetamine (AMPH) Action: Insights from Reserpine Studies and Genetic Manipulations

Fig no 3 Role of Plasma Membrane Uptake Transporters and Monoamine Secretory/Synaptic Vesicles in Amphetamine (AMPH) Action.
Initial studies using reserpine suggested a role for secretory vesicles in AMPH action. However, subsequent literature presented conflicting findings due to variations in experimental design [112]. Most studies, focusing on dopamine and norepinephrine systems, administered in vivo reserpine injections prior to AMPH administration. These studies generally found that reserpine blocked AMPH-mediated release of norepinephrine, suggesting involvement of vesicular catecholamine [113 ,114, 115]. However, the synthesis of norepinephrine from dopamine within vesicles may affect the interpretation of these results. Combinatorial studies on reserpine and AMPH effects on dopamine release yielded mixed results, with some experiments showing no effect of reserpine on AMPH, while others reported blockade. Attempts to resolve these discrepancies using synaptosomes also produced contradictory results [116, 117]. One explanation for these inconsistencies is that reserpine may upregulate tyrosine hydroxylase activity, leading to increased cytosolic dopamine levels. Shorter exposure to reserpine depleted exocytic dopamine release but did not increase tyrosine hydroxylase activity [118, 119]. This suggests that vesicular catecholamines contribute significantly to AMPH-mediated efflux under typical conditions [120]. Recent studies using genetic manipulations have shed light on the role of synaptic vesicles in AMPH action. Experiments with transfected cell lines and mouse mutants indicate the involvement of both dopamine transporter (DAT) and vesicular monoamine transporter (VMAT) in AMPH-mediated dopamine release [121, 122, 123]. Further investigations using electrochemical detection techniques and mutated transporters support the idea that AMPH acts on both vesicular and plasma membrane transporters, affecting monoamine pools in both synaptic vesicles and the cytosol. Additionally, AMPH-induced de novo dopamine synthesis may contribute to its effects [124, 125, 126]. Furthermore, AMPH affects both vesicular and cytosolic catecholamine pools, with implications for neurotransmitter release and cellular signaling. Its multifaceted actions highlight the complexity of its mechanism of action and its potential therapeutic implications [127, 128]. Amphetamines, including amphetamine and methamphetamine derivatives, exert their pharmacological effects through interactions with monoamine transporters and vesicular monoamine transporters (VMATs) within presynaptic neurons [129, 130]. This review delves into the intricate mechanisms by which amphetamines impact synaptic vesicle dynamics, focusing on neurotransmitter storage, release, and reuptake modulation [131, 132].
The impact on Amphetamines in Synaptic vesicles
Amphetamines are recognized psychostimulants that enhance monoaminergic neurotransmission by modulating monoamine transporters and VMATs. A comprehensive understanding of the interplay between amphetamines and synaptic vesicles is vital for comprehending their pharmacological implications and therapeutic potentiall [133, 134, 135].
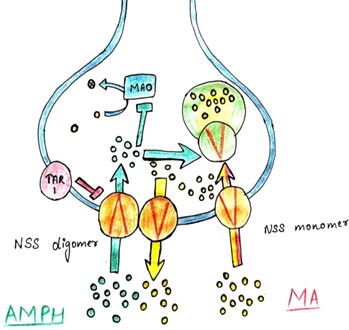
Fig.4. Mechanisms of Amphetamines in Synaptic vesicles
Monoamine Transporter Inhibition
Amphetamines function as substrates for monoamine transporters, such as the dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT) [136, 137]. By binding to and inhibiting these transporters, amphetamines impede the reuptake of monoamine neurotransmitters from the synaptic cleft into presynaptic neurons, thereby elevating extracellular monoamine levels, notably dopamine and norepinephrine [138, 139, 140].
Vesicular Monoamine Transporter (VMAT) Activation
In addition to monoamine transporter inhibition, amphetamines induce the reverse transport of monoamines by activating VMATs. VMATs play a pivotal role in packaging monoamine neurotransmitters into synaptic vesicles for storage and subsequent release. By facilitating the release of monoamines from synaptic vesicles into the presynaptic neuron's cytoplasm, amphetamines further augment extracellular monoamine concentrations [141, 142 , 143].
Disruption of Vesicular Storage
Extended exposure to high amphetamine concentrations can deplete monoamine stores within synaptic vesicles. This depletion results from VMAT reversal, which prompts the efflux of monoamines from synaptic vesicles into the cytoplasm, leading to a decline in vesicular monoamine content over time. Consequently, neurotransmitter release becomes less reliant on vesicular exocytosis and increasingly dependent on cytoplasmic reserves [144, 145, 146].
Increased Neurotransmitter Release
The cumulative effects of monoamine transporter inhibition and VMAT activation culminate in heightened release of monoamine neurotransmitters, particularly dopamine, into the synaptic cleft. This excessive neurotransmitter release intensifies monoaminergic signaling and contributes to the psychostimulant effects of amphetamines, including heightened arousal, euphoria, and enhanced cognitive function [147, 148, 149]. Lastly, Amphetamines intricately modulate synaptic vesicle function by influencing the dynamics of monoamine neurotransmitter storage, release, and reuptake. A nuanced comprehension of these mechanisms offers valuable insights into the pharmacological effects of amphetamines and holds promise for the development of innovative therapeutic strategies targeting monoaminergic neurotransmission [150, 151].
The impact on Amphetamines in Plasma membrane vesicles
Amphetamines (AMPH) are psychostimulant drugs that exert their pharmacological effects by interacting with plasma membrane transporters involved in the reuptake of monoamine neurotransmitters [152, 153]. This article aims to provide a comprehensive overview of AMPH actions on monoamine transporters, focusing on the dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT) [154].
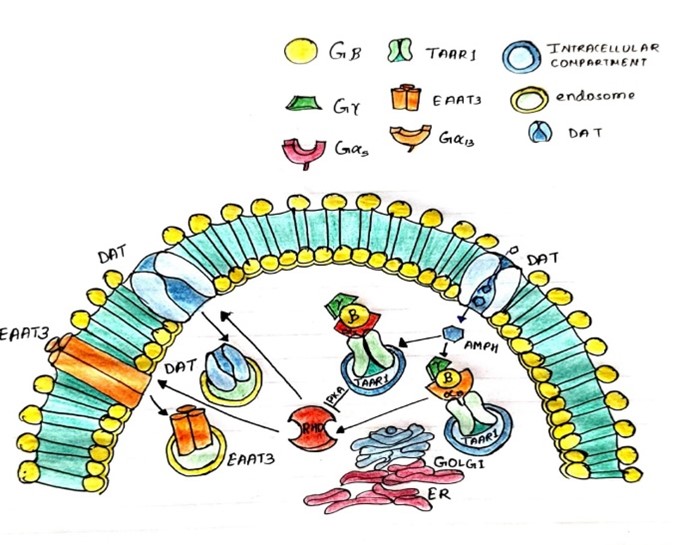
Fig.5. Mechanisms of Amphetamines in Plasma membrane vesicles
Substrate for Monoamine Transporters
AMPH compounds, including amphetamine and methamphetamine, serve as substrates for monoamine transporters. Structurally similar to endogenous neurotransmitters like dopamine, norepinephrine, and serotonin, AMPH molecules are recognized and actively transported by these transporters [155].
Reuptake Inhibition
Upon entering the presynaptic neuron, AMPH competitively inhibits the reuptake of monoamine neurotransmitters from the synaptic cleft into the presynaptic neuron. By binding to and blocking the activity of monoamine transporters, AMPH disrupts the normal reuptake process, leading to an accumulation of neurotransmitters in the synaptic cleft [156].
Enhanced Monoaminergic Signaling
The inhibition of monoamine reuptake by AMPH results in prolonged presence of neurotransmitters in the synaptic cleft, leading to enhanced monoaminergic signaling. This augmentation of neurotransmission contributes to the psychostimulant effects of AMPH, including increased arousal, euphoria, and improved cognitive function [157, 158, 159].
Dopamine Transporter (DAT) Interaction
AMPH exhibits high affinity for DAT, the primary transporter responsible for dopamine reuptake. Binding to DAT, AMPH inhibits dopamine reuptake, leading to elevated extracellular dopamine levels. This increase in dopamine neurotransmission is associated with the rewarding and reinforcing effects of AMPH [160, 161].
Norepinephrine Transporter (NET) Interaction
Similarly, AMPH interacts with NET, the transporter responsible for norepinephrine reuptake. By inhibiting NET activity, AMPH increases extracellular norepinephrine levels, contributing to its stimulant effects on arousal and attention [162].
Serotonin Transporter (SERT) Interaction
While less potent compared to its effects on DAT and NET, AMPH also interacts with SERT, leading to increased extracellular serotonin levels. This modulation of serotonin neurotransmission may contribute to the mood-elevating effects of AMPH [163, 164].
Additional Mechanisms Influencing Extracellular Catecholamine Levels
While amphetamine (AMPH) primarily interacts with plasma membrane transporters, it also influences extracellular catecholamine levels through alternative mechanisms. This section explores these additional pathways, offering insight into the broader regulatory network governing catecholamine neurotransmission [165, 166].
Vesicular Exocytosis and Secretory Vesicles
AMPH's impact extends beyond plasma membrane transporters to include vesicular exocytosis and secretory vesicles. By activating vesicular monoamine transporters (VMATs), AMPH facilitates the release of catecholamines stored within synaptic vesicles into the cytoplasm, a process known as reverse transport, thereby elevating extracellular catecholamine levels [167, 168, 169, 170].
Tyrosine Hydroxylase Activity
AMPH may modulate extracellular catecholamine levels by affecting tyrosine hydroxylase activity, the enzyme critical for catecholamine synthesis. Through enhanced tyrosine hydroxylase activity, AMPH promotes the conversion of tyrosine to L-DOPA, increasing substrate availability for catecholamine synthesis and subsequent release [171].
8Monoamine Oxidase Inhibition
Inhibition of monoamine oxidase (MAO), responsible for catecholamine degradation, represents another potential mechanism. By inhibiting MAO activity, AMPH extends the lifespan of extracellular catecholamines, leading to their accumulation in the synaptic cleft and amplifying neurotransmission [172, 173].
Neuronal Firing and Action Potential Generation
AMPH's stimulatory effects on neuronal firing and action potential generation indirectly influence extracellular catecholamine levels. Increased neuronal excitability promotes neurotransmitter release, including catecholamines, into the synaptic cleft, thereby augmenting extracellular concentrations [174, 175].
Glial Cell Interactions
Evidence suggests that glial cells, particularly astrocytes, play a role in regulating extracellular neurotransmitter levels. AMPH-induced alterations in glial cell function, such as calcium signaling and glutamate release, may indirectly impact catecholamine neurotransmission and contribute to changes in extracellular catecholamine levels [176, 177, 178]. In summary, AMPH's influence on extracellular catecholamine levels encompasses various mechanisms beyond plasma membrane transporters. Through modulation of vesicular exocytosis, tyrosine hydroxylase activity, MAO inhibition, neuronal firing, and glial cell interactions, AMPH exerts complex effects on catecholamine neurotransmission. Understanding these alternative mechanisms provides a comprehensive perspective on AMPH's pharmacological actions and offers potential insights for therapeutic interventions targeting catecholamine dysregulation [179, 180, 181].
CONCLUSION
This article provides a comprehensive exploration of the multifaceted mechanisms underlying the actions of amphetamines, focusing primarily on their impact on neurotransmission. By delving into the historical context, current research, and future directions, it sheds light on the intricate interplay between amphetamines and the central nervous system. The review carefully examines the structural diversity of amphetamines and their psychoactive effects, drawing parallels with the botanical diversity of orchids. It navigates through the elevation of extracellular catecholamines and serotonin levels, elucidating the mechanisms underlying these effects independently of classical transmitter release pathways. The cautious definition of "the amphetamines" encompasses compounds sharing a common structural motif, allowing for a focused discussion primarily on AMPH and methamphetamine. Subtle distinctions between these compounds are explored, alongside their historical utilization from natural plant-derived sources to contemporary clinical applications. Furthermore, the review investigates the role of trace amines in affective modulation and their potential as endogenous amphetamines, providing insights into the broader neurobiological landscape. It also traces the evolution and impact of synthetic amphetamines, from their inception to contemporary epidemics and clinical uses. Through a comprehensive exploration of amphetamine actions on synaptic vesicles and plasma membrane transporters, the article unravels their complex pharmacological effects and therapeutic implications. By dissecting the mechanisms underlying amphetamine-mediated neurotransmission, it offers valuable insights into both the rewarding and addictive nature of these compounds, as well as their potential therapeutic applications. Overall, this review contributes to our understanding of the multifaceted mechanisms of amphetamines and their impact on neurotransmission, paving the way for future research and clinical interventions in this field.
REFERENCES:
- Kennedy DO. Plants and the human brain. Oxford University Press, USA; 2014.
- Schmidt BM, Cheng DM, editors. Ethnobotany: A phytochemical perspective. John Wiley & Sons; 2017 Sep 25.
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Progress in neurobiology. 2005 Apr 1;75(6):406-33.
- Anderson BB, Chen G, Gutman DA, Ewing AG. Dopamine levels of two classes of vesicles are differentially depleted by amphetamine. Brain research. 1998 Mar 30;788(1-2):294-301.
- Benzenhöfer U, Passie T. Rediscovering MDMA (ecstasy): the role of the American chemist Alexander T. Shulgin. Addiction. 2010 Aug;105(8):1355-61.
- Shulgin AT. Drugs of abuse in the future. Clinical Toxicology. 1975 Jan 1;8(4):405-56.
- Sitte HH, Freissmuth M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends in pharmacological sciences. 2015 Jan 1;36(1):41-50.
- VM A. Comparative Study of Structural and Electronic Properties of Amphetamine Derivatives Using Density Functional Theory.
- Allen S. The Combined Neuropharmacology and Toxicology of Major'Bath Salts' Constituents MDPV, Mephedrone, and Methylone.
- Liu H, Zheng Y, Wang Y, Wang Y, He X, Xu P, Huang S, Yuan Q, Zhang X, Wang L, Jiang K. Recognition of methamphetamine and other amines by trace amine receptor TAAR1. Nature. 2023 Dec 21;624(7992):663-71.
- Kvernmo T, Härtter S, Burger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clinical therapeutics. 2006 Aug 1;28(8):1065-78.
- Spanagel R, Eilbacher B, Wilke R. Memantine-induced dopamine release in the prefrontal cortex and striatum of the rat—a pharmacokinetic microdialysis study. European journal of pharmacology. 1994 Sep 1;262(1-2):21-6.
- Gulley JM, Stanis JJ. Adaptations in medial prefrontal cortex function associated with amphetamine-induced behavioral sensitization. Neuroscience. 2010 Mar 17;166(2):615-24.
- Dent MF, Neill DB. Dose-dependent effects of prefrontal dopamine on behavioral state in rats. Behavioral neuroscience. 2012 Oct;126(5):620.
- Hall DA, Stanis JJ, Marquez Avila H, Gulley JM. A comparison of amphetamine-and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology. 2008 Jan;195:469-78.
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology. 2003 Feb;165:359-69.
- Logan BK. Methamphetamine-effects on human performance and behavior. Forensic Science Review. 2002 Jan;14(1):133-51.
- Ballesteros-Yáñez I, Castillo CA, Merighi S, Gessi S. The role of adenosine receptors in psychostimulant addiction. Frontiers in pharmacology. 2018 Jan 10;8:320873.
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. International review of neurobiology. 2009 Jan 1;88:101-19.
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol.. 2007 Feb 10;47:681-98.
- Appendino G, Minassi A, Taglialatela-Scafati O. Recreational drug discovery: natural products as lead structures for the synthesis of smart drugs. Natural product reports. 2014;31(7):880-904.
- Chambers SA, DeSousa JM, Huseman ED, Townsend SD. The DARK side of total synthesis: strategies and tactics in psychoactive drug production. ACS chemical neuroscience. 2018 Jan 17;9(10):2307-30.
- Freeman S, Alder JF. Arylethylamine psychotropic recreational drugs: a chemical perspective. European Journal of Medicinal Chemistry. 2002 Jul 1;37(7):527-39.
- Wang J, Yuan W, Li MD. Genes and pathways co-associated with the exposure to multiple drugs of abuse, including alcohol, amphetamine/methamphetamine, cocaine, marijuana, morphine, and/or nicotine: a review of proteomics analyses. Molecular neurobiology. 2011 Dec;44:269-86.
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the US population. Drug and alcohol dependence. 1997 Jan 10;44(1):11-29.
- Atzendorf J, Rauschert C, Seitz NN, Lochbühler K, Kraus L. The use of alcohol, tobacco, illegal drugs and medicines: an estimate of consumption and substance-related disorders in Germany. Deutsches Ärzteblatt International. 2019 Sep;116(35-36):577.
- Faro AF, Di Trana A, La Maida N, Tagliabracci A, Giorgetti R, Busardò FP. Biomedical analysis of New Psychoactive Substances (NPS) of natural origin. Journal of Pharmaceutical and Biomedical Analysis. 2020 Feb 5;179:112945.
- Morris JS, Groves RA, Hagel JM, Facchini PJ. An N-methyltransferase from Ephedra sinica catalyzing the formation of ephedrine and pseudoephedrine enables microbial phenylalkylamine production. Journal of Biological Chemistry. 2018 Aug 1;293(35):13364-76.
- Iqbal A, Khera RA, Hanif MA, Ayub MA, Zafar MN. Ma-Huang. InMedicinal Plants of South Asia 2020 Jan 1 (pp. 479-494). Elsevier.
- Rousseaux CG. Herbal Remedies. InHaschek and Rousseaux's Handbook of Toxicologic Pathology, Volume 3 2023 Jan 1 (pp. 183-303). Academic Press.
- Newton DE. Prescription drug abuse: a reference handbook. Bloomsbury Publishing USA; 2015 Dec 7.
- Haller CA, Jacob III P, Benowitz NL. Pharmacology of ephedra alkaloids and caffeine after single?dose dietary supplement use. Clinical Pharmacology & Therapeutics. 2002 Jun;71(6):421-32.
- Lee MR. The history of Ephedra (ma-huang). JR Coll Physicians Edinb. 2011 Mar 1;41(1):78-84.
- Sachs M. Ephedra and the failure of dietary supplement regulation. Cath. UL Rev.. 2004;54:661.
- Tynes JR. Performance enhancing substances: Effects, regulations, and the pervasive efforts to control doping in Major League Baseball.
- Palamar J. How ephedrine escaped regulation in the United States: a historical review of misuse and associated policy. Health Policy. 2011 Jan 1;99(1):1-9.
- Zusman MB. New Approaches to the Methamphetamine Epidemic.
- Patel NB. Mechanism of action of cathinone: the active ingredient of Khat (Catha Edulis. East African Medical Journal. 2000;77(6).
- Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert opinion on drug safety. 2005 Nov 1;4(6):1145-54.
- Klein A, Beckerleg S. Building castles of spit: The role of khat in work, ritual and leisure. InConsuming habits: Global and historical perspectives on how cultures define drugs 2014 Apr 8 (pp. 238-254). Routledge.
- Lemieux AM, Li B, al’Absi M. Khat use and appetite: an overview and comparison of amphetamine, khat and cathinone. Journal of ethnopharmacology. 2015 Feb 3;160:78-85.
- Weli AM, El-Shaibany A. Interference of khat with treatment of paranoid schizophrenia. Int J Chem Pharm Sci. 2011;2(1):1-1.
- Farah B. The health risks of khat and influences it has on integration issues. 2011.
- Alamgir, A.N.M. and Alamgir, A.N.M., 2017. Medicinal, non-medicinal, biopesticides, color-and dye-yielding plants; secondary metabolites and drug principles; significance of medicinal plants; use of medicinal plants in the systems of traditional and complementary and alternative medicines (CAMs). Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1: Pharmacognosy, pp.61-104.
- Bentley R. Secondary metabolites play primary roles in human affairs. Perspectives in biology and medicine. 1997;40(2):197-221.
- Pichini S, Marchei E, Palmi I, Pellegrini M, Pacifici R, Zuccaro P. Smart drugs. English edition. Istituto Superiore di Sanità. 2011.
- Funayama S, Cordell GA. Alkaloids: a treasury of poisons and medicines. Elsevier; 2014 Oct 21.
- Doesburg-van Kleffens M, Zimmermann-Klemd AM, Gründemann C. An overview on the hallucinogenic peyote and its alkaloid mescaline: the importance of context, ceremony and culture. Molecules. 2023 Dec 5;28(24):7942.
- Jenkins AJ, Gates MJ. Hallucinogens and psychedelics. Principles of forensic toxicology. 2020:467-89.
- Calina D, Carvalho F, Docea AO. Toxicity of psychedelic drugs. InToxicological risk assessment and multi-system health impacts from exposure 2021 Jan 1 (pp. 545-556). Academic Press.
- Axelrod J, Saavedra JM. Octopamine, phenylethanolamine, phenylethylamine and tryptamine in the brain. Aromatic amino acids in the brain. 1974 Jan 1:51-9.
- Pryor A, Hart S, Berry MD. Synthesis and neurochemistry of trace amines. InTrace Amines and Neurological Disorders 2016 Jan 1 (pp. 27-43). Academic Press.
- Quay WB. Catecholamines and tryptamines. Neurohormones and Neurohumors: Structure and Function of Regulatory Mechanisms. 1969:212-35.
- Brune GG. Biogenic amines in mental illness. International Review of Neurobiology. 1965 Jan 1;8:197-220.
- Berry M. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Reviews on recent clinical trials. 2007 Jan 1;2(1):3-19.
- Guzmán DC, Olguín HJ, Soto MP, Jiménez FT, Her-rera MO. Drug Interactions with Catecholamines and Metabolites. Evidence of Oxidative Stress and Inflammation On Pathways. J Neurosci Neuropsyc. 2022;5:103.
- Sotnikova TD, Budygin EA, Jones SR, Dykstra LA, Caron MG, Gainetdinov RR. Dopamine transporter?dependent and?independent actions of trace amine ??phenylethylamine. Journal of neurochemistry. 2004 Oct;91(2):362-73.
- Miner NB, Phillips TJ, Janowsky A. The role of biogenic amine transporters in trace amine–associated receptor 1 regulation of methamphetamine-induced neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 2019 Oct 1;371(1):36-44.
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biological psychiatry. 2005 Jun 1;57(11):1397-409.
- Sharma A. Investigating the Role of Parkin in Methamphetamine Use Disorder (Doctoral dissertation, Wayne State University).
- Lin J. Investigating the effects of methamphetamine on metabolite markers and white matter in the brain (Doctoral dissertation, ResearchSpace@ Auckland).
- Benedetti MS, Dostert P. Overview of the present state of MAO inhibitors. Monoamine Oxidase Enzymes: Review and Overview. 1987 Jan 1:103-19.
- Xie Z, Miller GM. ?-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: implication for modulatory roles of trace amines in brain. Journal of Pharmacology and Experimental Therapeutics. 2008 May 1;325(2):617-28.
- Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Frontiers in neuroscience. 2016 Apr 5;10:190244.
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang GE, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB. Amphetamine, 3, 4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Molecular pharmacology. 2001 Dec 1;60(6):1181-8.
- Ostovari H, Zahedi E, Sarvi I, Shiroudi A. Kinetic and mechanistic insight into the formation of amphetamine using the Leuckart–Wallach reaction and interaction of the drug with GpC· CpG base-pair step of DNA: a DFT study. Monatshefte für Chemie-Chemical Monthly. 2018 Jun;149:1045-57.
- Racine N. Blood, Meth, and Tears: The Super Soldiers of World War II.
- Rue HP, Espach RH. Refining of light petroleum distillates. US Government Printing Office; 1930.
- Larraz R. A brief history of oil refining. Substantia. 2021 Sep 9;5(2):129-52.
- Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present–a pharmacological and clinical perspective. Journal of psychopharmacology. 2013 Jun;27(6):479-96.
- Uddin MS, Sufian MA, Kabir MT, Hossain MF, Nasrullah M, Islam I, Mamun AA, Islam MT, Khanum S. Amphetamines: Potent recreational drug of abuse. J Addict Res Ther. 2017;8(4):1-2.
- Zaroslinski JF, Possley LH, Schwartz RA, Morris RN, Carone FA, Browne RK. The pharmacology and subacute toxicology of dopamine. Proceedings of the Royal Society of Medicine. 1977 Feb;70(2_suppl):2-6.
- McFadden Jr ER. A century of asthma. American journal of respiratory and critical care medicine. 2004 Aug 1;170(3):215-21.
- Grinspoon L, Hedblom P. The speed culture: Amphetamine use and abuse in America. Harvard University Press; 1975.
- Roseboom II PH. The effects of in vivo amphetamine treatment on dopamine receptor-coupled adenylate cyclase activities in the rat striatum. University of Michigan; 1989.
- Jackson CO. The amphetamine inhaler: a case study of medical abuse. Journal of the History of Medicine and Allied Sciences. 1971 Apr 1;26(2):187-96.
- Williams YD. Preclinical evaluation of lobeline for the treatment of ADHD: Comparison with psychostimulant therapies. University of Kentucky; 2011.
- Hando J. Patterns of illicit psychostimulant use in Australia and correlates of harm (Doctoral dissertation, UNSW Sydney).
- Ciccarone D. Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Primary Care: Clinics in Office Practice. 2011 Mar 1;38(1):41-58.
- Sevarino KA, Shelby BC. Stimulant Use Disorders. Psychiatry. 2015 Mar 23:1561-614.
- von Clausewitz C, Tzu S. Drugs in the Contemporary American Armed Forces. Shooting Up: A Short History of Drugs and War. 2016 Feb 10:263.
- NEUMEYER JL. of Medicinal Chemistry. Foye's Principles of Medicinal Chemistry. 2012 Jan 24:1.
- Edelstein MD. Zar spirit possession among Ethiopian Jews in Israel: Discourses and performances in religious identity, psychiatry, and public culture. Tulane University; 2001.
- Beck J. MDMA: The popularization and resultant implications of a recently controlled psychoactive substance. Contemp. Drug Probs.. 1986;13:23.
- Weisheit R, White WL. Methamphetamine: Its history, pharmacology and treatment. Simon and Schuster; 2009 Aug 19.
- Sequeira RP. Central nervous system stimulants and drugs that suppress appetite. InSide Effects of Drugs Annual 2012 Jan 1 (Vol. 34, pp. 1-16). Elsevier.
- DADKAR V, DADKAR N. GENERAL MEETING OF THE INDIAN PHARMACOLOGICAL SOCIETY.
- Barar FS. Essentials of pharmacotherapeutics. S. Chand Publishing; 2000.
- DUA P, DHAWAN B, SAXENA A. ANNUAL CONFERENCE OF THE INDIAN PHARMACOLOGICAL SOCIETY.
- Oliver G. The Croonian lectures: a contribution to the study of the blood and the circulation: delivered before the Royal College of Physicians of London. British medical journal. 1896 Jun 6;1(1850):1433.
- Savchuk T, Kovpak A, Semenenko N. History of adrenal discovery.
- Carmichael SW. The history of the adrenal medulla. Reviews in the Neurosciences. 1989 Jan;2(2):83-100.
- Starke K. History of catecholamine research. InHistory of Allergy 2014 (Vol. 100, pp. 288-301). Karger Publishers.
- Norrby E. Nobel prizes and notable discoveries. World Scientific; 2016 Sep 6.
- Adams C. Electromagnetic Health: Making Sense of the Research and Practical Solutions for Electromagnetic Fields (EMF) and Radio Frequencies (RF). Logical Books; 2024 Jan 30.
- Yamashima T. Adrenaline/Epinephrine Hunters: Past, Present and Future at 1900. Emerg Med Inves. 2017;4(4):EMIG-145.
- Elliott TR, Dale HH. THE DISCOVERY OF CHEMICAL NEUROTRANSMISSION. A History of the Brain: From Stone Age surgery to modern neuroscience. 2014 Dec 8:289.
- Rubin RP. A brief history of great discoveries in pharmacology: in celebration of the centennial anniversary of the founding of the American Society of Pharmacology and Experimental Therapeutics. Pharmacological reviews. 2007 Dec 1;59(4):289-359.
- Buccino RA, Sonnenblick EH, COOPER T, BRAUNWALD E. Direct positive inotropic effect of acetylcholine on myocardium. Circulation research. 1966 Dec;19(6):1097-108.
- Freye E. Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs: A comprehensive review on their mode of action, treatment of abuse and intoxication.
- DeVido JJ. Stimulants: Caffeine, Cocaine, Amphetamine, and Other Stimulants. Absolute Addiction Psychiatry Review: An Essential Board Exam Study Guide. 2020 Mar 7:185-203.
- Weiss RD, Mirin SM, Bartel RL. Cocaine. American Psychiatric Pub; 2002 Nov 1.
- Triarhou LC. The pioneer neuropharmacologist Alfred Fröhlich (1871–1953) and the origins of neuroendocrinology: a sesquicentennial remembrance. The Neuroscientist. 2023 Feb;29(1):19-29.
- Triarhou LC. Alfred Fröhlich (1871–1953). InThe Brain Masters of Vienna: Psychology and Neuroscience Pioneers around the Secession up to the Anschluss 2022 Oct 21 (pp. 137-141). Cham: Springer International Publishing.
- Alper MH, Flacke W, Krayer O. Pharmacology of reserpine and its implications for anesthesia. Anesthesiology. 1963 Jul 1;24(4):524-42.
- Sharma MK, Kumar S, Sharma MK. PHARMACOLOGICAL PROPERTIES OF RAUVOLFIA SERPENTINA.
- Starke K. History of catecholamine research. InHistory of Allergy 2014 (Vol. 100, pp. 288-301). Karger Publishers.
- Carlsson A. The discovery of the SSRIs: a milestone in neuropsychopharmacology and rational drug design. Selective serotonin reuptake inhibitors: Past, present and future. 1999:1-7.
- Bhagat B, Shideman FE. Mechanism of the inhibitory action of guanethidine on cardiovascular responses to tyramine and amphetamine. Journal of Pharmacology and Experimental Therapeutics. 1963 Jun 1;140(3):317-23.
- Innes IR, Karr GW. Protection against induction of supersensitivity to catecholamines by cocaine. British Journal of Pharmacology. 1971 Aug;42(4):603-10.
- Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. Dopamine neuronal transport kinetics and effects of amphetamine. Journal of neurochemistry. 1999 Dec;73(6):2406-14
- Lotharius J, Brundin P. Impaired dopamine storage resulting from ?-synuclein mutations may contribute to the pathogenesis of Parkinson's disease. Human molecular genetics. 2002 Oct 1;11(20):2395-407.
- Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Molecular neurobiology. 2009 Apr;39:73-80.
- Støier JF, Konomi-Pilkati A, Apuschkin M, Herborg F, Gether U. Amphetamine-induced reverse transport of dopamine does not require cytosolic Ca2+. Journal of Biological Chemistry. 2023 Aug 1;299(8).
- Kumar N. Neurotransmitter Systems: Dopamine. The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine. 2019:29-51.
- Michaelis ML, Michaelis EK, Myers SL. Adenosine modulation of synaptosomal dopamine release. Life sciences. 1979 May 28;24(22):2083-92.
- Reith ME, Gnegy ME. Molecular mechanisms of amphetamines. Substance Use Disorders: From Etiology to Treatment. 2020:265-97.
- Mandela P, Chandley M, Xu YY, Zhu MY, Ordway GA. Reserpine-induced reduction in norepinephrine transporter function requires catecholamine storage vesicles. Neurochemistry international. 2010 May 1;56(6-7):760-7.
- Harsing Jr L. Dopamine and the dopaminergic systems of the brain. Handbook of neurochemistry and molecular neurobiology: Neurotransmitter systems. 2008 Apr 15:155.
- Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux: a voltage-sensitive and intracellular Na+-dependent mechanism. Journal of Biological Chemistry. 2003 Apr 4;278(14):12070-7.
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. Journal of Neuroscience. 1998 Mar 15;18(6):1979-86.
- Wheeler DS, Underhill SM, Stolz DB, Murdoch GH, Thiels E, Romero G, Amara SG. Amphetamine activates Rho GTPase signaling to mediate dopamine transporter internalization and acute behavioral effects of amphetamine. Proceedings of the National Academy of Sciences. 2015 Dec 22;112(51):E7138-47.
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LF, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proceedings of the National Academy of Sciences. 2000 Jun 6;97(12):6850-5.
- Lohr KM, Masoud ST, Salahpour A, Miller GW. Membrane transporters as mediators of synaptic dopamine dynamics: implications for disease. European Journal of Neuroscience. 2017 Jan;45(1):20-33.
- Belovich AN. Reverse Transport of the Dopamine Transporter in Neuropsychiatric Disease: Mechanisms of Regulation and Dysfunction. Vanderbilt University; 2017.
- Fon EA, Edwards RH. Molecular mechanisms of neurotransmitter release. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2001 May;24(5):581-601.
- Mosharov EV, Gong LW, Khanna B, Sulzer D, Lindau M. Intracellular patch electrochemistry: regulation of cytosolic catecholamines in chromaffin cells. Journal of Neuroscience. 2003 Jul 2;23(13):5835-45.
- Goldstein DS. Neurocardiology: therapeutic implications for cardiovascular disease. Cardiovascular therapeutics. 2012 Apr;30(2):e89-106.
- Magee CP. Psychostimulants and Monoaminergic Transporter Function (Doctoral dissertation, The University of Utah).
- Liu Y, Peter D, Merickel A, Krantz D, Finn JP, Edwards RH. A molecular analysis of vesicular amine transport. Behavioural brain research. 1995 Dec 15;73(1-2):51-8.
- Sahai MA, Opacka-Juffry J. Molecular mechanisms of action of stimulant novel psychoactive substances that target the high-affinity transporter for dopamine. Neuronal Signaling. 2021 Dec;5(4):NS20210006.
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug and alcohol dependence. 1998 Jun 1;51(1-2):87-96.
- German CL, Baladi MG, McFadden LM, Hanson GR, Fleckenstein AE. Regulation of the dopamine and vesicular monoamine transporters: pharmacological targets and implications for disease. Pharmacological reviews. 2015 Oct 1;67(4):1005-24.
- Benarroch EE. Monoamine transporters: structure, regulation, and clinical implications. Neurology. 2013 Aug 20;81(8):761-8.
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Molecular neurobiology. 2009 Apr;39:149-70.
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC pharmacology. 2006 Dec;6:1-7.
- Zhou J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs of the Future. 2004 Dec;29(12):1235.
- Xing B, Li YC, Gao WJ. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain research. 2016 Jun 15;1641:217-33.
- Murray TF. Biogenic Amine Transmitters. Veterinary Psychopharmacology; Wiley: Omaha, NE, USA. 2019 Jan 3:29-42.
- Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annual review of pharmacology and toxicology. 2003 Apr;43(1):261-84.
- Riddle EL, Fleckenstein AE, Hanson GR. Role of monoamine transporters in mediating psychostimulant effects. The AAPS journal. 2005 Dec;7:E847-51.
- Ayala-Lopez N, Watts SW. Physiology and pharmacology of neurotransmitter transporters. Compr. Physiol. 2021 Jun 30;11:2279-95.
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997 Dec 1;19(6):1271-83.
- Wallace LJ. Effects of amphetamine on subcellular distribution of dopamine and DOPAC. Synapse. 2012 Jul;66(7):592-607.
- Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harbor perspectives in biology. 2012 Feb 1;4(2):a005595.
- Lawal HO, Krantz DE. SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine. Molecular aspects of medicine. 2013 Apr 1;34(2-3):360-72.
- Ebenezer I. Neuropsychopharmacology and therapeutics. John Wiley & Sons; 2015 Aug 3.
- Pasternak GW. Monday, December 4, 2006 Panel Session Co-Morbid Pain and Addiction: Novel Treatments. Neuropsychopharmacology. 2006;31:S1-69.
- Steinkellner T, Freissmuth M, Sitte HH, Montgomery T. The ugly side of amphetamines: short-and long-term toxicity of 3, 4-methylenedioxymethamphetamine (MDMA,‘Ecstasy’), methamphetamine and D-amphetamine.
- Di Giovanni G, Svob Strac D, Sole M, Unzeta M, Tipton KF, Mück-Šeler D, Bolea I, Della Corte L, Nikolac Perkovic M, Pivac N, Smolders IJ. Monoaminergic and histaminergic strategies and treatments in brain diseases. Frontiers in neuroscience. 2016 Nov 24;10:541.
- Huang M, He W, Rajagopal L, Kudwa A, Grigoriadis DE, Meltzer HY. Effects of NBI-98782, a selective vesicular monoamine transporter 2 (VMAT2) inhibitor, on neurotransmitter efflux and phencyclidine-induced locomotor activity: Relevance to tardive dyskinesia and antipsychotic action. Pharmacology Biochemistry and Behavior. 2020 Mar 1;190:172872.
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. European journal of pharmacology. 2003 Oct 31;479(1-3):23-40.
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nature Reviews Neuroscience. 2003 Jan;4(1):13-25.
- Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslén B, Marsal K, Hansson SR. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004 Jul 1;25(6):518-29.
- Sitte HH, Freissmuth M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends in pharmacological sciences. 2015 Jan 1;36(1):41-50.
- Deutch AY, Roth RH. Pharmacology and biochemistry of synaptic transmission: classical transmitters. InFrom Molecules to Networks 2014 Jan 1 (pp. 207-237). Academic Press.
- Asser A, Taba P. Psychostimulants and movement disorders. Frontiers in neurology. 2015 Apr 20;6:127637.
- Rizzo FR, Bruno A, Federici M, Mercuri NB. 3, 4-Methylenedioxymethamphetamine (MDMA) and Synaptic Dopamine. InHandbook of Substance Misuse and Addictions: From Biology to Public Health 2022 Jun 7 (pp. 1-19). Cham: Springer International Publishing.
- Teixeira-Gomes A, Costa VM, Feio-Azevedo R, de Lourdes Bastos M, Carvalho F, Capela JP. The neurotoxicity of amphetamines during the adolescent period. International Journal of Developmental Neuroscience. 2015 Apr 1;41:44-62.
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. The Journal of Neuroscience. 2001 May 5;21(9):RC141.
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse?. Neuroscience & biobehavioral reviews. 2006 Jan 1;30(2):215-38.
- Jamkhande PG, Khawaja A. Role of norepinephrine reuptake inhibitors in attention deficit hyperactivity disorder: A mechanism-based short review. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2016 Oct 1;6(4):146-51.
- Barbier AJ, Aluisio L, Lord B, Qu Y, Wilson SJ, Boggs JD, Bonaventure P, Miller K, Fraser I, Dvorak L, Pudiak C. Pharmacological characterization of JNJ-28583867, a histamine H3 receptor antagonist and serotonin reuptake inhibitor. European journal of pharmacology. 2007 Dec 8;576(1-3):43-54.
- Wenthur CJ, Bennett MR, Lindsley CW. Classics in chemical neuroscience: fluoxetine (Prozac). ACS chemical neuroscience. 2014 Jan 15;5(1):14-23.
- Cameron KN, Solis Jr E, Ruchala I, De Felice LJ, Eltit JM. Amphetamine activates calcium channels through dopamine transporter-mediated depolarization. Cell Calcium. 2015 Nov 1;58(5):457-66.
- M Chiu V, O Schenk J. Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Current drug abuse reviews. 2012 Sep 1;5(3):227-42.
- Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harbor perspectives in biology. 2012 Feb 1;4(2):a005595.
- Chen R, Furman CA, Gnegy ME. Dopamine transporter trafficking: rapid response on demand. Future neurology. 2010 Jan;5(1):123-34.
- Alter SP. Impaired vesicular storage of norepinephrine and serotonin in Parkinson's disease-related pathogenesis (Doctoral dissertation, Emory University).
- Westerink RH. Targeting exocytosis: ins and outs of the modulation of quantal dopamine release. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2006 Feb 1;5(1):57-77.
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Archives of biochemistry and biophysics. 2011 Apr 1;508(1):1-2.
- Stefanovic B, Spasojevic N, Jovanovic P, Jasnic N, Djordjevic J, Dronjak S. Melatonin mediated antidepressant-like effect in the hippocampus of chronic stress-induced depression rats: Regulating vesicular monoamine transporter 2 and monoamine oxidase A levels. European neuropsychopharmacology. 2016 Oct 1;26(10):1629-37.
- Vecchio LM. Investigating Monoamine Regulation: The Transport Of The Dopamine Transporter To Striatal Terminals And The Effects Of Tyrosine Hydroxylase Overexpression On Dopaminergic and Noradrenergic-Related Phenotypes. University of Toronto (Canada); 2016.
- Pocock G, Richards CD. Excitatory and inhibitory synaptic mechanisms in anaesthesia. BJA: British Journal of Anaesthesia. 1993 Jul 1;71(1):134-47.
- HF Vandael D, Marcantoni A, Carbone E. Cav1. 3 channels as key regulators of neuron-like firings and catecholamine release in chromaffin cells. Current Molecular Pharmacology. 2015 Aug 1;8(2):149-61.
- Finkbeiner SM. Glial calcium. Glia. 1993 Oct;9(2):83-104.
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Diaz-Cabiale Z, Rivera A, Ferraro L, Tanganelli S, Tarakanov AO, Garriga P, Narváez JA, Ciruela F. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal–glial networks. Frontiers in physiology. 2012 Jun 4;3:136.
- Van Calker D, Hamprecht B. Effects of neurohormones on glial cells. InAdvances in cellular neurobiology 1980 Jan 1 (Vol. 1, pp. 31-67). Elsevier.
- Guzmán DC, Olguín HJ, Soto MP, Jiménez FT, Her-rera MO. Drug Interactions with Catecholamines and Metabolites. Evidence of Oxidative Stress and Inflammation On Pathways. J Neurosci Neuropsyc. 2022;5:103.
- Perry ML. Fast Capillary Electrophoresis for the Study of Dopamine in the Central Nervous System (Doctoral dissertation).
- Couto L. Methamphetamine-Induced DNA Double-Stranded Breaks: The Impact of the Dopamine Transporter and Insights Into the Mechanisms of DNA Damage in Mouse Neuro 2a Cells.


 Arnab Roy*
Arnab Roy*
 Mahesh Kumar Yadav 2
Mahesh Kumar Yadav 2
 Abrarul Haque 3
Abrarul Haque 3
 Pratik Mondal
Pratik Mondal
 Gautam Mahto 5
Gautam Mahto 5
 Balraj Kumar 5
Balraj Kumar 5
 Nisha Kumari 5
Nisha Kumari 5
 Bina Kumari 5
Bina Kumari 5
 Maheshwar Kumar 4
Maheshwar Kumar 4
 Sunny Kumar 5
Sunny Kumar 5
 Abhishek Kumar 4
Abhishek Kumar 4
 Shweta Kumari
Shweta Kumari
 K. Rajeswar Dutt 1
K. Rajeswar Dutt 1







 10.5281/zenodo.11142830
10.5281/zenodo.11142830