Abstract
Novel Drug Delivery Systems (NDDS) can rejuvenate existing drug molecules, enhancing their market value, competitiveness, and extending their patent life. Glibenclamide (Glyburide), a BCS Class II drug, has a short elimination half-life (2-5 hours), requiring twice-daily dosing, which may lead to non-compliance. Its solubility is improved by forming a complex with ?-Cyclodextrin via the kneading method, showing increased solubility in a 1% w/v ?-Cyclodextrin solution (0.0002630 mg/ml) compared to lower concentrations. Controlled porosity osmotic pump (CPOP) tablets of Glyburide were developed and evaluated for pre- and post-compression parameters. The tablets were coated with a semi-permeable membrane using a 10% w/v Opadry enteric coat in isopropyl alcohol, with Polyethylene Glycol-400 as a plasticizer. Optimization was achieved using a 3² factorial design and ANOVA for quality assessment. Formulation F6, with a 10% coating solution, high NaCl, and medium tartaric acid, exhibited slow drug release over 20 hours, rapid swelling, and minimal erosion, independent of the release media's pH. FTIR studies confirmed that all drug absorption peaks were retained in the formulation. DSC overlay indicated a shift to higher temperatures, suggesting some interaction between the drug and polymers. The optimized formulation remained stable after short-term accelerated stability studies.
Keywords
Novel Drug Delivery System, Controlled porosity Osmotic System, Glibenclamide, Glyburide
Introduction
In recent years, there has been significant progress in the development of Novel Drug Delivery Systems (NDDS). This shift is primarily driven by the relatively lower development costs and shorter timeframes required to introduce NDDS compared to developing entirely new chemical entities. NDDS can rejuvenate existing drug molecules, enhancing their market value, competitiveness, and improving patient outcomes.1
One of the most promising NDDS advancements is the development of Oral Osmotic Pumps. Over the past two decades, various types of oral osmotic delivery systems have been created and studied for drugs with differing aqueous solubility’s. Conventional drug delivery systems often lack precise control over drug release, making it difficult to achieve effective concentrations at the target site, resulting in unpredictable plasma levels. In contrast, oral controlled drug delivery systems offer a more consistent and prolonged drug release, allowing for predictable drug absorption rates and extents.2
However, traditional controlled release (CR) systems, such as matrix or reservoir types, can exhibit bioavailability fluctuations due to changes in gastric pH and the body's hydrodynamic conditions. Osmotic systems, on the other hand, provide drug release that is independent of these physiological factors. Drug release from osmotic systems is governed by osmosis, which is unaffected by environmental pH. These systems can take various forms, including implants and tablets, and are activated by physical, chemical, or biochemical processes.3
There are over 240 patented osmotic drug delivery systems, commonly referred to as Gastro-Intestinal Therapeutic Systems (GITS). Today, numerous osmotic pumps designed for various drugs are available to meet patient needs and requirements. In a typical therapeutic regimen, drug dose and dosing intervals are optimized to maintain drug concentration within the therapeutic window, ensuring efficacy while minimizing toxic effects. Controlled drug delivery systems provide spatial control over drug release, and osmotic pumps are among the most promising for this purpose. Osmotic pumps operate on the principle of osmosis. They consist of an inner core containing the drug and osmogens, coated with a semi-permeable membrane. As the core absorbs water, it expands, pushing the drug solution out through delivery ports. The drug release rate from osmotic pumps is independent of the pH and hydrodynamics of the dissolution medium. Various patented osmotic drug delivery systems include the Rose-Nelson pump, Higuchi-Leeper pump, Higuchi-Theeuwes pump, and the elementary osmotic pump.4
Controlled release dosage forms are designed to release drugs in vivo at a predictable rate that can be verified by in vitro measurements. Potential developments and new approaches to oral controlled release dosage forms include:
- Hydrodynamic Pressure Controlled System: Utilizes the body's natural hydrodynamic pressures to control drug release.
- Intragastric Floating Tablet: Remains buoyant in the stomach to prolong drug release time.
- Transmucosal Tablet: Facilitates drug absorption through the mucosal lining.
- Microporous Membrane Coated Tablet: Uses a microporous membrane to regulate drug release.
These innovations in controlled drug delivery systems hold great promise for improving therapeutic outcomes and patient adherence to treatment regimens.
Osmotic drug delivery has made significant advancements since the pioneering work of Australian physiologists Rose and Nelson, who developed the first implantable pump in 1955. This method leverages osmotic pressure for controlled drug delivery using osmogens, which can maintain drug release for up to 10–16 hours. The current research focuses on developing an osmotic drug delivery system for Glibenclamide, a sulfonylurea-class antidiabetic medication. Glibenclamide, which belongs to BCS Class II, has an elimination half-life of 2-5 hours, necessitating three daily doses for many patients. This frequent dosing schedule often leads to noncompliance. Diabetes mellitus is a chronic metabolic disorder characterized by high blood glucose levels due to insulin deficiency or resistance. Glibenclamide is an oral hypoglycemic agent used to treat patients with Non-Insulin Dependent Diabetes Mellitus (NIDDM). It works by inhibiting ATP-sensitive potassium channels in pancreatic beta cells. This inhibition leads to cell membrane depolarization, opening voltage-dependent calcium channels, which increases intracellular calcium levels in beta cells and stimulates insulin release.
The proposed work aims to carry out the preformulation, optimization, and development of a controlled porosity osmotic pump for the poorly soluble drug Glibenclamide (also known as Glyburide). This system aims to eliminate the need for complicated and expensive drilling techniques. In these systems, osmotic pressure is generated by osmotic agents, and polymer swelling forces drive the drug out through pores created by pore-forming agents. The pores form as the pore-forming agents dissolve when the system is exposed to water. An osmotic tablet of Glibenclamide fits the concept of improved patient compliance, delayed release, enhanced efficacy, and sufficient bioavailability to achieve the desired pharmacological action with fewer gastrointestinal side effects. The development of this controlled porosity osmotic pump is expected to provide a more convenient and effective treatment option for patients with NIDDM, addressing the challenges associated with frequent dosing and ensuring better therapeutic outcomes.
MATERIALS AND METHODS:
MATERIALS:
The materials for this study were sourced from well-regarded suppliers to ensure quality. Glibenclamide and ?-Cyclodextrin were procured from Yarrow Chem in Mumbai. HPMC K100 M came from Colorcon, Asia Pvt. Ltd. in Goa. Excipients and solvents such as microcrystalline cellulose, iso-propyl alcohol, Carbopol 934, magnesium stearate, talc, aerosil, NaCl, and tartaric acid were supplied by LobaChemie in Mumbai.
METHODS:
Characterisation of Drug:
The characterization of Glibenclamide (Glyburide) involved evaluating its organoleptic properties, including color, odor, and appearance. The melting point was determined using a Thiel’s tube apparatus, where a small amount of the drug was placed in a capillary tube and heated in a paraffin bath until it melted. For solubility testing, excess Glibenclamide was added to 5 mL of various solvents- distilled water, 0.1 N HCl, phosphate buffers at pH 6.8 and 7.4, and methanol and shaken in a rotary shaker at room temperature for 24 hours.5-7
FTIR absorption spectrum of Glibenclamide:
FT-IR spectrum of drug sample was recorded as potassium bromide (KBr) powder at resolution of 4cm-1 over the region of 4000-400 cm-1 for its authentication and to study principle peaks using FT-IR spectrophotometer (? C FT-IR, Bruker). Dry sample of drug and potassium bromide was mixed uniformly and filled into the die cavity of sample holder and an IR spectrum was recorded. The identified peaks were compared with the principle peaks of reported IR spectrum and the sample was authenticated.8
Differential scanning calorimetry of Glibenclamide:
The DSC pattern was recorded on a PerkinElmer 4000, Software: PYRIS Version-11.1.0.0488, 2009, PerkinElmer, Inc. Glibenclamide (1.0 mg ) was heated in crimped aluminium pan with a pierced lid at a scanning rate of 100C/min an atmosphere of nitrogen purging performed and flow (20ml/min) using the range of 30-3500C. The DSC was calibrated for baseline using empty pans, and for temperature and enthalpy using indium.8
Preformulation Study:
Selection and Characterization of Polymers:
Selection of Polymers:
The polymers were selected on basis of its melting point and molecular weight. Also compatibility of polymers with the drug was studied before. The release pattern was decided first according to that the polymers were selected to extend the release of drug.7
The method used to formulate inclusion complex are as follow:
Kneading Technique :
In this technique, cyclodextrin (CD) is added with isopropyl alcohol and converted to paste. Drug is then added and kneaded for specified time. The kneaded mixture is then dried and passed through sieve if required.9-19
Preparation and Evaluation of factorial Batches for Selection of Concentration of Sustain Release agent:
Glibenclamide and polymer blends were prepared as per the compositions reported and pass through sieve no 40. Tablets were prepared on rotary punching machine by wet granulation method. The compression pressure was adjusted to obtain tablet with hardness in range of 8-10 kg/cm2. 20-22
Factorial Design :
A 32 factorial design was implemented for optimization of Osmotic tablet formulation of Glibenclamide. According to the model it contained 2 independent variables at 3 levels, +1,0,-1. According to model total 9 formulations are possible, the composition of different formulation are shown in table 1 The different independent variables, were conc. of OsmogenNaCl (X1) and Tartaric acid(X2). Dependent factors included % drug release and % Wt Gain after coating at 12 hrs.23-25
Factorial batches F1-F9 were prepared as per the composition reported in table8.3all preliminary batches in second lot were prepared same as lot first. In these preliminary batches concentration of HPMC and water swellable polymers were changed.
Table 1: Translation of coded value in actual unit

Table 2: Factorial design for preparation of batches F1-F9
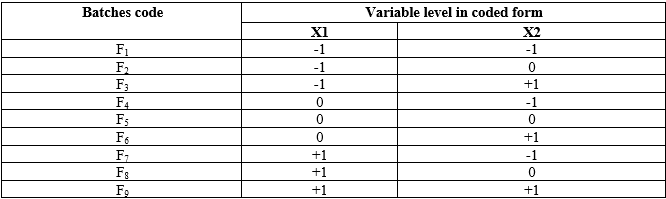
Table 3: Composition and their concentration (mg) in factorial batches F1-F9
Preparation of Glibenclamide controlled porosity osmotic pump tablet
Preparation of Core Tablet:
The preparation of the core tablet involved several key steps. First, all solid ingredients were sieved through a 60-mesh sieve and accurately weighed according to the quantities specified. Next, a complex was formed by weighing the specified amounts of PVPK30, ?-Cyclodextrin, and the drug, which were then mixed using isopropyl alcohol via the kneading method for 30 minutes.26-30 For the wet granulation process, all ingredients except the lubricant were combined using the dry mix blend method with HPMC K100, Carbopol 934, microcrystalline cellulose (MCC), NaCl, and tartaric acid. This mixture was granulated and the wet mass was then dried at 50°C in an oven until the loss on drying (LOD) was below 2% w/w, as determined by a halogen moisture detector at 105°C. The dried granules were sifted through a 40-mesh sieve. The granules were then lubricated with magnesium stearate, which had been sieved through a 60-mesh sieve, and mixed for 5 minutes at 24 rpm in an octagonal blender. Finally, the lubricated granules were compressed into tablets using 9.0 mm round standard concave punches in a rotary compression machine.31-34
Coating Process:
The core tablets were coated using a conventional coating pan machine. The coating process involved an inlet air temperature of 50°C, a bed temperature of 38°C, a pan speed of 6 to 8 rpm, and an atomization air pressure of 2.0 psi. The average tablet weight was monitored periodically to achieve the desired weight gain of 5% to 10%. The coated tablets were then dried at 50°C for 30 minutes in the coating pan at 1-2 rpm. The coating solution was prepared by dissolving Opadry enteric coat and polyethylene glycol 400 in isopropyl alcohol with constant stirring.35
Coating of tablet core:
Tablet core formulation F6 was selected to optimize the coating solution formula. Isopropyl alcohol is used as solvents, PEG 400 as plasticizer, Core tablets were coated with coating solution in an automatic perforated coating pan. Initially, pan was rotated at low speed (3-5 rpm) and heated air was passed through tablet bed. Coating process was started only after outlet temperature was reached to 30ºC. Coating pan rpm was maintained in the range of 15-20 rpm and coating solution was applied at the rate of 5-7 ml/min. coating process was continued until desired weight was gained on tablet core. For all formulations, coated tablets were dried at 40ºC for 3 hours before evaluation. After coating dry tablets were weighed for percentage weigh gain up to 12 % by following equation.36
% weigh gain = (Wt-Wo/ Wo) *100
Where, Wt = weight of tablet after coating
Wo = weight of tablet before coating
After coating tablets were in vitrorelease study of tablets was tested for 12 hour by using 6.8 phosphate buffers.
Evaluation of Factorial Batches:37-40
Evaluation of Powder Characteristics:
The powder properties include bulk density (BD), tap density (TD), Hausner ratio (HR), Carr’s index (CI) were determined using Tap density tester. The powder sample under test was screened through sieve no. 40 and weighed sample was transferred in 50ml cylinder. The mechanical tapping of cylinder was carried out using tapped density tester. Initially volume, (Vo) was noted to the nearest graduated unit. Calculate the bulk density, in gm/ml by the formula. Tapping was continued further for an additional tapping and tapped volume (Vb) was noted.
A) Bulk Density:
It is the ratio of total mass of powder to the bulk volume of powder. It was measured by pouring the weighed powder into a measuring cylinder and the initial volume was noted. This initial volume is called the bulk volume. From this, the bulk density is calculated according to the formula mentioned below. It is expressed in g/cc and is given by:
Bulk density =Mass of powder/Bulk volume
B) Tap Density:
It is the ratio of total mass of powder to the tapped volume of powder. The volume was measured by tapping the powder for 50 times. Then the tapping was done for 100 times and the tapped volume was noted. It is expressed in g/cc and is given by:
Tap density =Mass of powder /Tapped volume
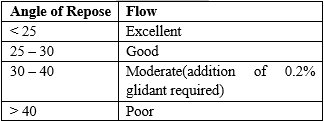
C) Angle of Repose:
Weighed quantity of microspheres was passed through a funnel fixed on a stand at a specific height upon graph paper. A static heap of powder with only gravity acting upon it was tending to form a conical mound. The height of the heap (h) and radius (r) of lower part of cone were measured. The angle of repose was calculated using formula:
?=tan-1 (h/r)
Where, ? = angle of reponse, H = height of the cone, r = radius of the cone base.
Table 4: Relationship between flow properties and angle of repose
D) Hausner’s ratio:
Hausner ratio of microparticles was determined by comparing the tapped density to the bulk density using the equation
Hausner’s ratio =Tapped density/Bulk density
E) Carr’s Index or % compressibility:
The Carr’s index was determined from the tapped density and poured density (bulk density) as per the formula (USP, 2004).
Carr’s index (%) = Tap density- bulk density/Tap density × 100
Table 5: Relationship between flow properties and Carr’s Index

Table 6: Relationship between flow properties and Hausner ratio

Evaluation of Tablet characteristics:
All tablets were tested for appearance, colour and odour.
A) Tablet thickness
Thickness of tablet is important for uniformity of tablet size. Thickness was measured in mm using Vernier Caliper. It was determined by checking ten tablets from each formulation.
F) Coating Thickness
It was measured by Vernier caliper and compared it with the thickness of core tablets.
B) Tablet hardness
The resistance of tablet to shipping or breakage, under conditions of storage, transportation and handling before usage depends on its hardness. The hardness of tablet of each formulation was measured by LABINDIA TH 1050 M. The hardness was measured in terms of kg/cm2. For each batch three tablets were tested.
C) Friability
Friability is the measure of tablet strength. Roche friabilator (FT1020, Labindia) was used for testing the friability using the following procedure.
Procedure: Twenty tablets were weighed accurately and placed in the tumbling apparatus that revolves at 25 rpm dropping the tablets through a distance of six inches with each revolution. After 100 revolutions, the tablets were weighed and the % friability was calculated measured using the following formula.
Friability = Initial weight of tablets - Final weight of tablets/ Initial weight of tablets
× 100
D) Weight Variation Test
Twenty tablets were randomly selected from each batch and individually weighed, by using Electronic balance. The average weight and standard deviation of twenty tablets were calculated.
PD=(average weight)-(individual weight)/(average weight)*100
Where, PD= Percentage deviation; W avg wt.= Average weight of tablet; W individual weight of tablet.
E) Drug content estimation
The Glibenclamide tablets were tested for their drug content. Five tablets were finely powdered; quantities of the powder equivalent to 10 mg of Glibenclamide were accurately weighed and transferred to a 100-ml of volumetric flask. The flask was filled with Phosphate buffer (pH 7.4) solution and mixed thoroughly. The solution was made up to volume and filtered. Dilute 10 ml of the resulting solution to 200 ml with Phosphate buffer (pH 7.4) and measure the absorbance of the resulting solution at the maximum at 229 nm using a Shimadzu UV/Vis double beam spectrophotometer. The linearity equation obtained from calibration curve as described previously was use for estimation of in the Glibenclamide tablet formulations.
F) Swelling Index
The swelling response of CPOTs containing drug were studied by hydration study. The formulations of tablets were weighed individually to determine initial weight of each tablets using electronic balance. They are placed in separate petridishes containing 10 ml water and are kept at room temperature. The tablets were removed from the petridishes at regular interval and water in excess was carefully blotted and the swollen tablets were weighed and replaced to the petridishes. This process is continued for 12 hours and the percentage of water uptake (WU%) were calculated using the equation.
WU% = (weight of tablet after swelling - initial weight)/ initial weight × 100
Optimization Data Analysis:41-44
Effect of independent variables on Drug Release:
The data obtained after evaluation of factorial batches was analysed by using commercially available software Design Expert version 9.0.4.1. To describe the response surface curvature, the design was evaluated by quadratic model, which bears the form of equation and result are reported.
Y= b0 + b1X1 + b2X2 +b3 X1X2 +b4X12 +b5X22
Where,
Y is the response variable,
b0 the constant, an arithmetic mean of all responses
b1, b2 the regression coefficient,
b4, b5 the regression coefficients show linearity.
X1 and X2 stand for the main effect, represent the average result of changing one factor at a time from its low to high value. The interaction terms X1 X2 demonstrate how the response changes when 2 factors are changed simultaneously. X21 X22 is used to investigate nonlinearity.
The relationship between the dependent and independent variables was further elucidated using response plots. Response plots help to logically predict values of the ?R and swelling index from formulating products.
RESULT AND DISCUSSION:
Characterization of the drug:
The characterization of the drug revealed that the sample was a white, amorphous, odorless powder. The melting point of plain Glibenclamide was determined to be 170°C, consistent with reported values. In terms of solubility, Glibenclamide was found to be soluble in acetone, methanol, and ethanol, slightly soluble in buffers at pH 6.8 and pH 7.4, and practically insoluble in water and hexane.
FTIR spectrum of drug:
The IR spectrum (figure 1) of the given sample of Glibenclamide showed similar characteristics peaks to that of reported spectrum of Glibenclamide. The FTIR spectrum of Glibenclamide was interpreted with the following observations:
The vibrational frequency at 3363.6948 cm??1; corresponds to C=O stretching, typically in the range of 3300-3600 cm??1;. A frequency of 682.4321 cm??1; matches C-Cl stretching, which is usually found between 600-800 cm??1;. The 746.2160 cm??1; frequency aligns with -NH stretching reported between 700-900 cm??1;. SO2 stretching was observed at 1338.1097 cm??1;, within the expected range of 1150-1350 cm??1;. Lastly, the Ar-H stretching was seen at 1611.6919 cm??1;, falling within the reported range of 1650-1420 cm??1;.
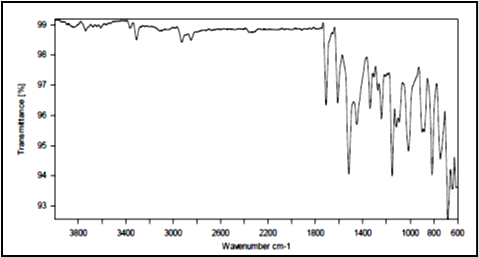
Figure 1: FTIR of Glibenclamide
Differential scanning calorimetry of Glibenclamide
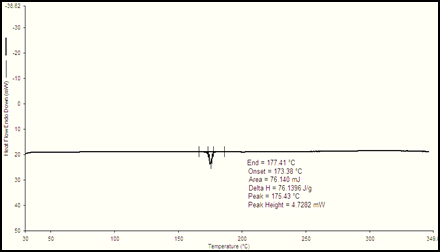
Figure 2: DSC of Glibenclamide
DSC thermogram of given sample of Glibenclamide was shown in figure 9.2.The thermogram shows small endothermic peak at 173.380Cand exothermic peak at 177.410 C. Sharp peak at 175.430 C which is near to the actual melting point of Glibenclamide. From this it was confirms that the given drug sample was Glibenclamide in crystalline nature at its exothermic peak.
Preformulation Study:
Selection of Polymers:
The polymers were selected on the basis of its melting point and molecular weight and its water swelling ability. Also compatibility of polymers with the drug was studied before. The release pattern was decided first as zero order release according to that the polymers were selected to extend the release of drug. As HPMC K 100M, Osmogen as NaCl and tartaric acid,Carbapol 934 were selected.
Solubility study:
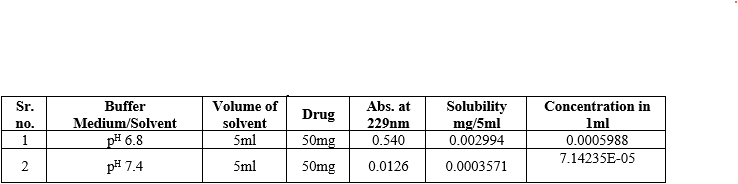
Solubility of Glibenclamide is more in 1%w/v [0.0002630mg/ml] solution of -ß-cyclodextrinamong 0.25%, 0.50% and 0.75%w/v solution. Solubility of Glibenclamide in distilled water is found to be 0.000206mg/ml.
- Solubility studies in buffer media pH6.8 and pH7.4
Table 7:- Solubility studies in Various Buffer media
Solubility studies of Glibenclamide: ß-Cyclodextrin complex in Phosphate buffer pH7.4:
Table 8: - Solubility studies in ß-Cyclodextrin solutions
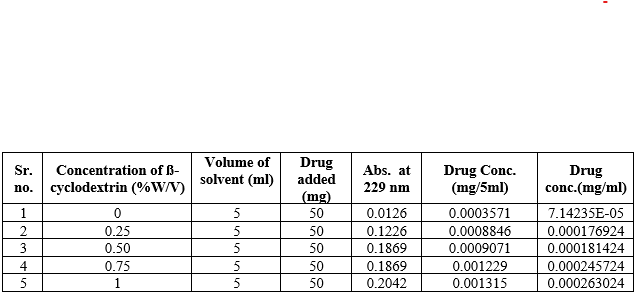
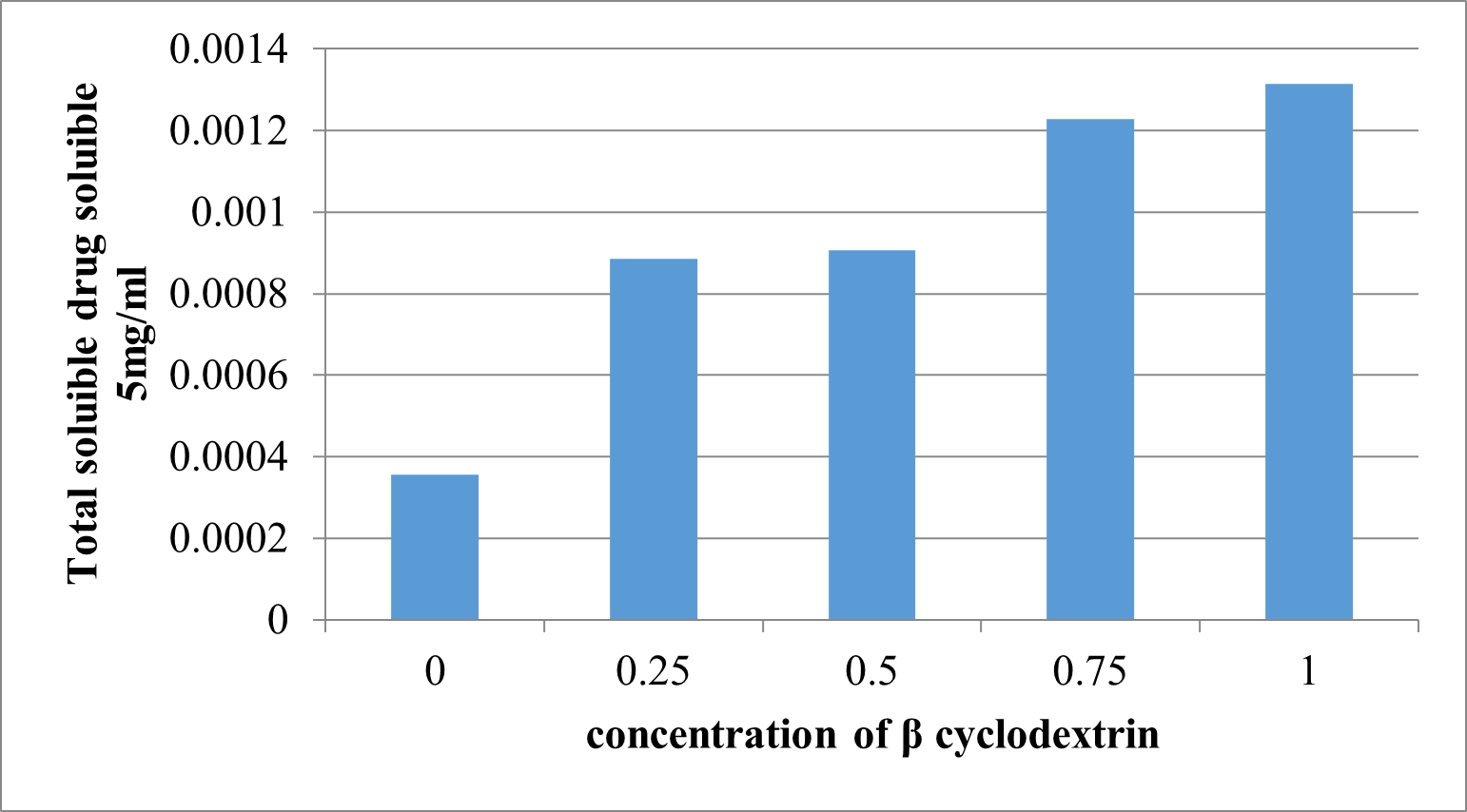
Figure 3: phase solubility graph of ?-CD in Phosphate buffer pH 7.4
Evaluation of powder flow properties:
Granules prepared for wet granulation method was evaluated by measuring the parameters such as; bulk density, angle of repose, Hausner’s factor, compressibility index and drug content. The table 9 presents the pre-compressive parameters of various tablet formulations (F1 to F9). It includes measurements for the angle of repose, loose bulk density, tapped density, Hausner factor, and Carr’s index, each provided with a standard deviation (S.D.). The angle of repose values range from 28.21±0.91 to 29.38±0.91, indicating good flow properties. Loose bulk density values vary slightly between 0.30±0.0 and 0.34±0.0 g/ml, while tapped density values range from 0.34±0.0 to 0.39±0.0 g/ml. The Hausner factor, indicating flowability, is consistently around 1.19±0.0 across all formulations. Carr’s index, which measures compressibility, ranges from 15.80±0.5% to 16.68±0.7%, suggesting acceptable compressibility characteristics for all tablet formulations.
Table 9: Values of pre-compressive parameters of tablet
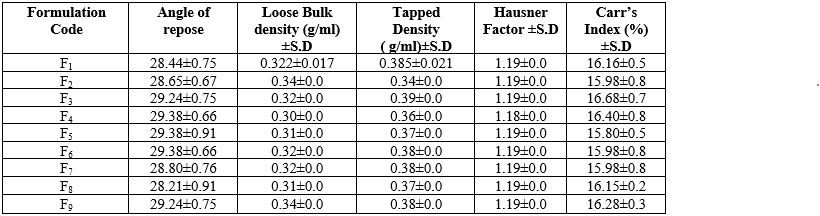
*All values was expressed as Mean ± SD (n=3)
Physical characteristics:
The physical characteristics of the prepared Controlled Porosity Osmotic Pump (CPOP) tablets, expressed as mean ± standard deviation (n=3), show varied results across different formulations (F1 to F9). The hardness of the tablets ranged from 5.6 to 6.36 Kg/cm?2;, with F1 showing the highest hardness at 6.36 Kg/cm?2; and F3 the lowest at 5.6 Kg/cm?2;. Weight variation was minimal, with all formulations close to the target weight, ranging from 293.33 mg to 295.66 mg. Friability, indicating tablet durability, was generally low, ranging from 0.51% to 0.57%, with F5 exhibiting the lowest friability at 0.51%. Drug content across the formulations varied slightly, with values ranging from 95.1% to 99.55%, with F7 having the highest drug content at 99.55% and F4 the lowest at 95.1%. These results suggest consistent physical properties and satisfactory drug content across all formulations.
Table 10: Physical characteristics of prepared CPOP Tablet
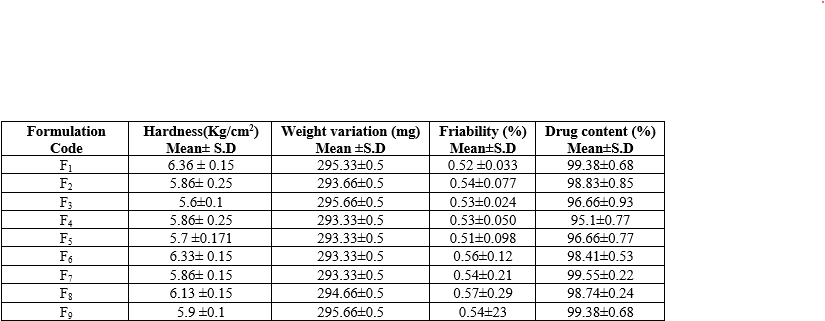
*All values was expressed as Mean ± SD (n=3)
Thickness:
The thickness of both uncoated and coated tablets was measured for various formulation batches (F1 to F9), expressed as mean ± standard deviation. The uncoated thickness of the tablets ranged from 2.95 mm to 4.47 mm, with F7 having the thinnest tablets at 2.95 mm and F5 the thickest at 4.47 mm. After coating, the thickness increased, with coated tablets ranging from 5.36 mm to 5.47 mm. The differences in thickness after coating reflect the application of a uniform coating layer, enhancing the overall consistency of the tablets. For instance, the coated thickness of F1 was 5.39 mm compared to its uncoated thickness of 4.38 mm, showing a consistent coating application. This data indicates that all batches maintained uniformity in both uncoated and coated states, ensuring reliable dosage form characteristics.
Table 11: Tablet thickness uncoated and coated thickness
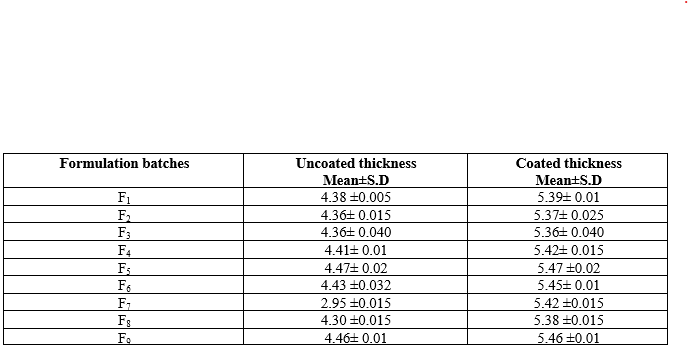
*All values was expressed as Mean ± SD (n=3)
Swelling index:
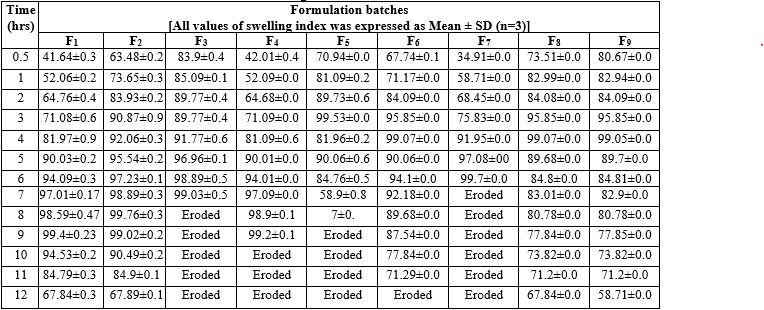
The swelling index of various factorial batches (F1 to F9) was measured over a 12-hour period, with values expressed as mean ± SD (n=3). Initially, at 0.5 hours, swelling indices ranged from 34.91±0.0 for F7 to 83.9±0.4 for F3. As time progressed, the swelling indices generally increased, reaching a peak around 7 to 8 hours for most formulations. For instance, F2 and F3 showed high swelling indices of 98.89±0.3 and 99.03±0.5, respectively, at 7 hours. F6 exhibited rapid swelling and minimal erosion, maintaining its integrity up to 10 hours. In contrast, some formulations like F3, F5, and F7 eroded by the 8th or 9th hour. The results indicated that F6 formulation, with a higher concentration of coating agents (NaCl and PEG400), demonstrated superior swelling properties and structural stability. This can be attributed to the thicker coat formed, which facilitated water imbibition inside the semi-permeable membrane (SPM).
Table 12: swelling index of factorial batches
In vitro drug release study:
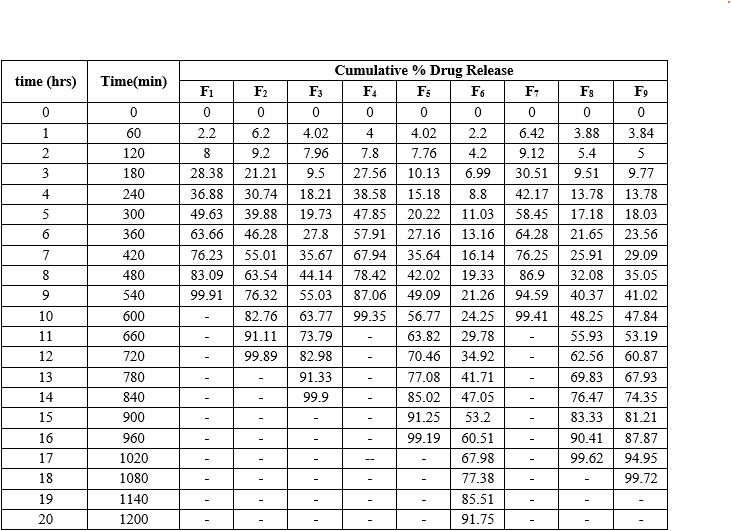
Formulation F1 to F9 all shows different result because of different concentrations of polymers used. As concentration of NaCl and Tartaric acid were changed, the drug release affected and gets changed. From the below observations it was concluded that the formulation F6 exhibited swelling and release characteristics in acceptable range. Hence, it was taken further for optimization study. In the above observations of % drug release, F3, f5, f6, f8 and f9 Formulation shows the drug release after 12 hrs time interval.AS % drug releases, F6 Formulation shows the 91.75% drug release at 20 hrs time interval. It was the maximum % drug release than other three formulations. From that it was concluded that the because concentration of osmotic agents NaCl, Tartaric acid and polymers which was added in the F1,f2 and F7 formulation % drug release observed lower than F6,f8 and F9.
The drug release profiles of various formulations were analyzed over a period of time. Formulation F1, which included PVPK30, Carbapol 934, HPMC K100, and MCC but low concentrations of NaCl and tartaric acid, achieved a maximum drug release of 99.91% within 9 hours. Formulations F2 and F3, with medium and high concentrations of NaCl, demonstrated drug releases of 99.89% and 99.90% within 12 and 14 hours, respectively. Formulation F4, containing low NaCl and medium tartaric acid, showed a slightly lower drug release of 99.35% within 10 hours. Formulation F5, with medium concentrations of both NaCl and tartaric acid, achieved a drug release of 99.19% within 16 hours. Formulation F6, with medium-low tartaric acid and high NaCl, showed a drug release of 91.75% over 20 hours. Finally, formulation F7, which had high tartaric acid and low NaCl, reached a drug release of 99.41% within 10 hours. Formulation F8, which included a high concentration of tartaric acid and a medium concentration of NaCl, achieved a drug release of 99.62% within 17 hours. Similarly, formulation F9, containing high concentrations of both tartaric acid and NaCl, showed a drug release of 99.72% within 18 hours.
Table 13: Cumulative (%)Drug Release of factorial batches (F1-F9)
Optimization Data Analysis:
Effect of independent variables on Drug Release
The polynomial equation obtained was
Y?R= +62.73 – 9.21X1-27.37X 2-2.17X1X2 +15.40 X1 2+11.21X22
Yswelling index=29.14222-1.53X1 -1.521X2+31.63X1X2 +43.71X1 2 -24.15X2 2
From the regression equation of the ?R, a negative sign indicates an increase the concentration of polymers it decreases the ?R and positive sign indicates that increases the concentration of polymers it increases the ?R. From the regression equation of swelling index a negative sign indicates an increase the concentration of polymers it decreases the hardness and positive sign indicates that increases the concentration of polymers it increases the swelling index. The interaction terms showed how the response changes when two factors were simultaneously changed. The relationship between the dependent and independent variables was further elucidated using contour plots.
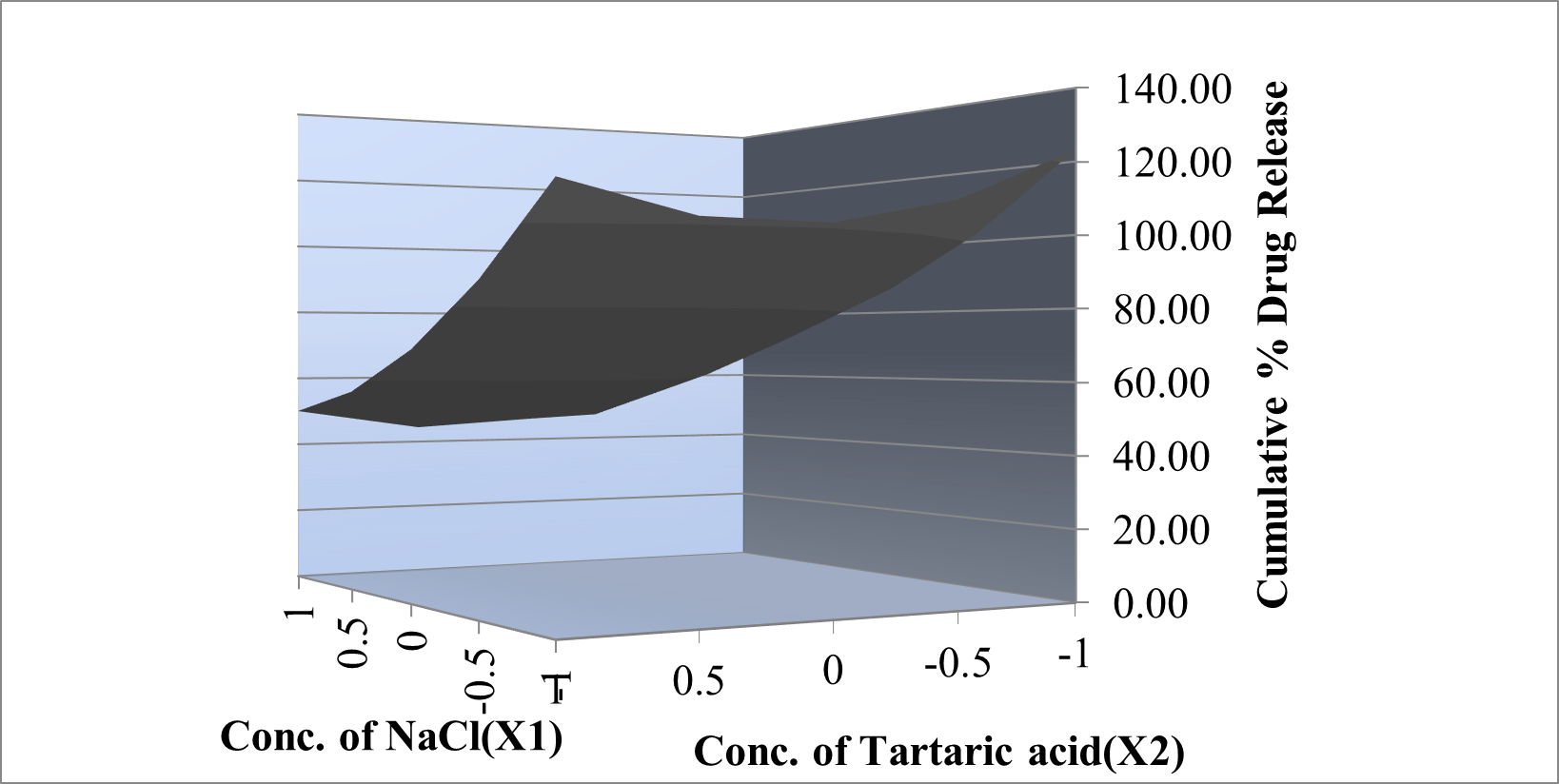
Figure 4: Response plot of ?R
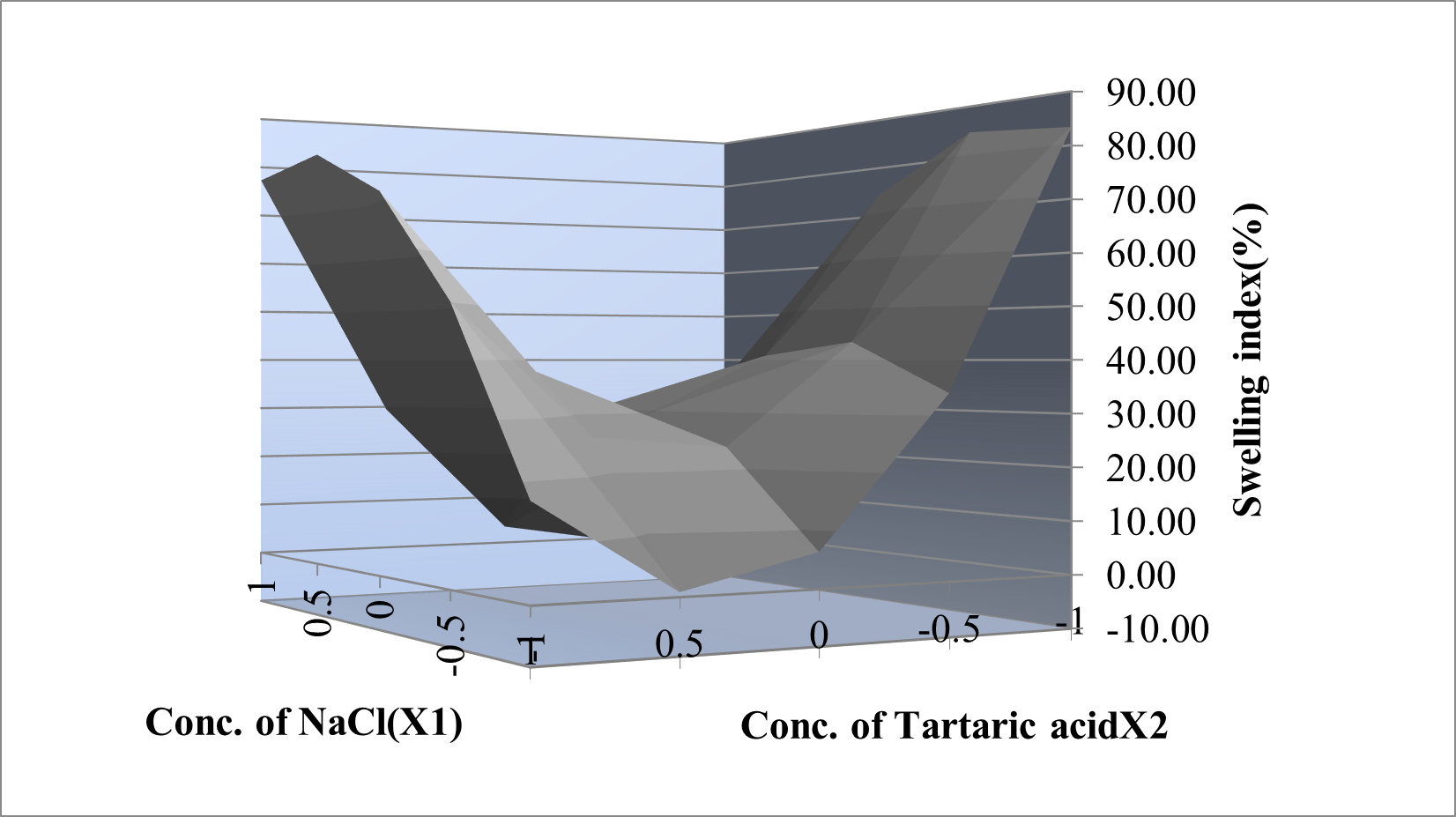
Figure 5: Response plot of Swelling index(%)
Treatment of Quality Assessment Techniques:
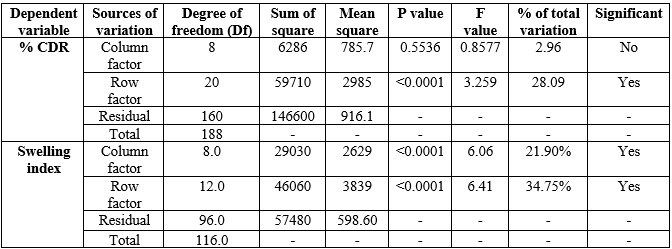
ANOVA Test:
From following ANOVA table 14, the treatment sum of squares is a measure of treatment differences, here large sum of squares means that treatment differences are large (&CDR and swelling index). Mean squares are variance estimates and higher values indicate random variation in groups (?R and swelling index). The p ? 0.05 suggests the terms of significance. The results of statistical analysis show that the p values of ?R show the value more than 0.05 for column (0.55) and less than row factors (<0>
Table 14: Two-way ANOVA of dependent variables
Stability Study for optimized batch:
Accelerated stability testing was carried out for the optimized formulation. The results of stability testing are as depicted in table15. Accelerated stability data obtained for optimized formulation revealed that organoleptic characteristics, hardness, Weight, drug content were within the acceptable limit. Thus the formulation can be said to be stable.
Table 15: Evaluation of Formulation F6 for accelerated Stability Testing

CONCLUSION
The research explores the design and evaluation of osmotically controlled tablets for Glibenclamide using NaCl and tartaric acid. Chapters I and II cover the introduction and objectives. Glibenclamide, a BCS Class II drug with low solubility and bioavailability, was formulated with ?-Cyclodextrin to enhance solubility. Osmotic Drug Delivery Systems (ODDS) are designed to improve controlled drug release by creating osmotic pressure. The study aimed to develop tablets with slow, prolonged drug release using NaCl as the osmotic agent. The research included preformulation studies and compatibility tests between the drug and polymers, with formulations evaluated for coating thickness and drug release. Out of nine formulations, F6, with 10% coating solution, demonstrated the best performance, showing fast swelling and high drug release. NaCl was found to provide better osmotic pressure compared to tartaric acid. The tablets exhibited good hardness, uniformity, and swelling properties. Analytical studies confirmed the drug's purity, and FTIR showed interactions between the drug and excipients. Solubility studies revealed that ?-Cyclodextrin improved Glibenclamide solubility. The drug content was consistent, and formulations met acceptable pre- and post-compression standards. Formulation F6, with high NaCl concentration, achieved a 91.75% drug release over 20 hours, following zero-order kinetics. Statistical analysis highlighted significant effects of formulation factors on drug release and swelling. Future work will focus on in-vivo studies of the formulation.
FUNDING:
Nil
AUTHORS CONTRIBUTIONS:
All authors have contributed equally.
CONFLICTS OF INTERESTS:
All authors have declared no conflict of interest.
REFERENCE
- Sancheti V, Chordiya M, Senthilkumaran K. A review on osmotically controlled drug delivery systems. World J Pharma. 2003;3(12):1708-1728.
- Srikanth P, Narayana R, Wasim S, Brito R. A review on oral controlled drug delivery systems. Int J Advanced Pharma. 2013;1(3):51-58.
- Nikam PH, Kareparambanj A, Jadhav AP, Kadam VJ. Osmotic pump: A reliable drug delivery system. Res J Pharma Bio Chem Sci. 2012;3(3):478.
- Mane SS, Kamble SM, Chaudhari P, Bhosle A. Review article: An overview on oral osmotically controlled drug delivery systems. Int J Universal Sci Life Sci. 2012;2(2).
- Singh MS, Pancholi SS. Comparative studies on dissolution enhancement of glibenclamide in solid dispersions made by different techniques. Int J Pharmaceutical Erudition. 2012;1(4):33-42.
- Dhillon B, Goyal NK, Sharma PK. Formulation and evaluation of glibenclamide solid dispersion using different methods. Global J Pharmacology. 2014;8(4):551-556.
- Gianotto EA, Saraiva AC. Dissolution test for glibenclamide tablets. Quim Nova. 2007;30(5):1218-1221.
- Khare NK, Banweer J, Tahilani P, Goyanar G. Formulation, development, and evaluation of an osmotic drug delivery system of glibenclamide. IJPSR. 2015;11:119.
- Nikhil S, Seema P. Enhancement of solubility of acyclovir by solid dispersion and inclusion complexation methods. World Appl Sci J. 2010;11(7):857-864.
- Dattatreya V, Hiremath SN. Formulation and in-vitro evaluation of buccoadhesive tablets containing ketoconazole inclusion complex with ?-cyclodextrin. 2009;2(2):396-404.
- Dhananjay G, Premachand DN. Preparation and characterization of domperidone inclusion complex with cyclodextrin: Influence of preparation method. Iranian J Pharm Res. 2009;8(3):145-151.
- Sanae M, Moulay Abbes F. Inclusion complex of hydrochlorothiazide-gamma-cyclodextrin: The effect on aqueous solubility, dissolution rate, bioavailability, and the effect on intestinal permeability using a chamber technique. Int J Pharm Pharma Sci. 2013;5(3):718-724.
- Camelia N, Cornia A. Phase solubility studies of the inclusion complex of repaglinide with ?-cyclodextrin and ?-cyclodextrin derivative. 2010;58(5):620-628.
- Manoj N, Dinesh S. The cyclodextrin: A review. J Current Pharma Res. 2012;10(1):1-6.
- Agrawal R, Gupta V. Cyclodextrins – A review on pharmaceutical applications for drug delivery. IJPFR. Jan-Mar 2012;2(1):95-112.
- Chaudhary VB, Patel JK. Cyclodextrin inclusion complex to enhance solubility of poorly water-soluble drugs: A review. IJPSR. 2013; 4(1):68-76.
- Review on various techniques for solubilization of poorly soluble drugs. Internationale Pharmaceutica Sciencia. July-September 2012;2(3):28-35.
- Eapen C, Prasanth V, Rai A. Development of UV spectrometric method for glibenclamide (glyburide) in bulk and pharmaceutical formulations. Int J Chem Tech Res. 2012;4(1):356-360.
- Camelia N, Cornia A. Phase solubility studies of the inclusion complex of repaglinide with ?-cyclodextrin and ?-cyclodextrin derivative. 2010;58(5):620-628.
- Himansu Bhusan S, Jitendra D. Solubility and dissolution improvement of aceclofenac using ?-cyclodextrin. Int J Drug Dev Res. 2012;4(4):326-333.
- Sreenivasa R, Mohammed M. Preparation and evaluation of cyclodextrin inclusion complex of water-insoluble drug glimepiride. Int J Res Pharma Biomed Sci. 2012;3(1):428-434.
- Vivekanand C, Abhishek M. Zaltoprofen ?-CD inclusion complex for solubility enhancement. J Pharm Sci Tech. 2013;3(1):37-42.
- The Indian Pharmacopoeia. The Controller of Publications. 2007;1:182-183.
- Lachman L, Lieberman H. The Theory and Practice of Industrial Pharmacy. 1991; 3:67-71, 183-184.
- Martindale W, Reynolds J. Martindale: The Extra Pharmacopoeia. The Pharmaceutical Press, London. 1996;31:936-937.
- Bahri-N, Tavakoli N, Senemar M, Peikanpour M. Preparation and pharmaceutical evaluation of glibenclamide slow release buccal film. 2013.
- Sharkheliya DB, Sharma R, Rajawat SG. Formulation development and assessment of controlled release bilayered osmotic tablet carrying sulfonylurea class – anti-diabetic agent & imperative factors imparting significant impact on drug release. Int J Res Pharm Sci. 2013; 3(2):102-122.
- Sastri P, Ravikumar, Kalra A, Kanagale A. Enhancement of dissolution of glipizide from controlled porosity osmotic pump using a wicking agent and a solubilizing agent. Int J Pharm Tech Res. 2009;1(3):705-711.
- Bharadwaj P, Upadhyay PK, Agarwal V, Chaurasia D, Chaurasia H, Singh R. Development and characterization of elementary osmotic pump tablets for simultaneous release of metformin and glipizide. Indian Drugs. 2012;49(11).
- Mahalaxmi R. Enhancement of dissolution of glipizide from controlled porosity osmotic pump using a wicking agent and a solubilizing agent. Int J Pharm Tech Res. 2009;1(3):705-711.
- Vyas SP, et al. Modified push-pull osmotic system for simultaneous delivery of theophylline and salbutamol: Development and in-vitro characterization. Int J Pharm. 2004;284:95-108.
- Mina Rani, et al. Development and biopharmaceutical evaluation of osmotic pump tablets for controlled delivery of diclofenac sodium. Acta Pharm. 2003;53:263-273.
- Jayaprakash S, Halith S. Formulation and evaluation of bilayer tablets of amlodipine besilate and metoprolol succinate. Der Pharmacia Lettre. 2011;3(4):143-154.
- Mishra R. Plasticizer effect and comparative evaluation of cellulose acetate and ethyl cellulose-HPMC combination coatings as semipermeable membranes for oral osmotic pumps of naproxen sodium. Drug Dev Ind Pharm. 2002;28(4):403-412.
- Patel BR, Patel HR, Patel GN. Development of osmotically controlled drug delivery system by inclusion complex with HP-?-CD of glipizide: Optimization of formulation using response surface methodology. Asian J Pharm Sci. 2010;5(2):74-86.
- Shirsand S, Swamy P. Design and evaluation of atenolol bilayer buccal tablets. J Pharm Sci. 2011;1(1):4-10.
- Brahmankar DM. Biopharmaceutics and Pharmacokinetics: A Treatise. 2009;2:430-450.
- Kuchekar B, Singavi A, et al. Indian Drugs. 2003;40(1):44-45.
- Brahmankar DM. Biopharmaceutics and Pharmacokinetics: A Treatise. 2009; 2:430-450.
- Sudha T, Krishna V, Kumar VR. Development and validation of an analytical method for glyburide and its related compounds in tablet formulation by HPLC-UV. Turk J Pharm Sci. 2014; 11(3):307-316.
- Avula PR, Veesam H. Influence of dependent variables on granule formulation using factorial design: Microwave irradiation as one of the factors. Int J Pharm Res. 2013;2(7):115-118.
- Khan MA. Formulation of sustained release diltiazem hydrochloride matrix tablets through optimization and their evaluation. J Pharm Bio Chem Res. 2013;4(2):1317-1325.
- Alexander M, Saini G. Factorial design used in optimization of immediate release solid dosage Ranitidine HCl. Estud Biol. 2006;28(62):17-25.
- Sharma S, Singh G. Formulation design and optimization of mouth dissolving tablets of domperidone using sublimation technique. Int J Pharm Sci. 2010;1(1):128-135


 Vishal R. Rasve* 7
Vishal R. Rasve* 7




















 10.5281/zenodo.13219968
10.5281/zenodo.13219968