Abstract
Periodontal disease, commonly known as gum disease, encompasses a range of inflammatory conditions affecting the tissues surrounding the teeth. The initial stage, gingivitis, is characterized by gum inflammation without bone loss. If untreated, it progresses through early, moderate, and advanced stages, potentially leading to severe periodontitis. The microbiology and pathophysiology of periodontal disease involve complex interactions between bacterial plaque and the host immune response, with various risk factors such as smoking, diabetes, and genetic predisposition contributing to disease onset and progression. Supportive therapy and drug delivery systems, both local and systemic, play crucial roles in maintaining periodontal health. Additionally, the side effects of conventional antibiotics necessitate exploring alternative treatments like herbal medicine. Recent advancements include the use of herbal medications, which offer potential benefits due to their anti-inflammatory and antimicrobial properties. However, challenges such as standardization, efficacy, and patient compliance remain. Herbal medications in periodontal therapy present a promising alternative, addressing some challenges associated with current management strategies. They offer fewer side effects compared to traditional antibiotics and can enhance patient outcomes. Nevertheless, rigorous clinical studies are needed to validate their effectiveness and integrate them into mainstream periodontal treatment protocols. So, the present review tries to briefly assess and describe the current role Using therapeutic plants in the process of mitigation and management for the relief from disease affecting periodontal disease.
Keywords
Periodontal Disease, Herbal medicatons, Anti-inflammatory Antimicrobial
Introduction
Bad oral hygiene and its negative impact creates a significant cost on individuals, healthcare systems, and societies globally. Public health has been greatly affected by the changing demographics and ageing population, mostly through an increase in the prevalence of chronic illnesses and impairments. [1,2] Dental and oral health are directly linked to overall health and are some of the most prevalent health issues that people face. [3] Even Nevertheless, there is currently little attention paid to oral health, despite the global focus on the chronic health issues associated with an ageing population. [4] The Global Burden of Disease 2010 Study found that oral health problems were responsible for 15 million years of disability-adjusted life years, or 224 years of health loss on average per 100,000 people [5].
It has been shown that oral disorders could potentially impact as many as 3.5 billion individuals globally. [2] According to WHO data on oral conditions the prevalence of declining oral health in both adults and children is steadily rising. Almost 530 million children worldwide have primary tooth decay, and between 60 to 90 percent of school-age children have caries, with Asian and Latin American countries having the highest rates of the disease. [6] The systemic and local health of people is continuously challenged by a multitude of infectious diseases.
Information derived from the 2009–2014 National Health and Nutrition Examination Survey
showed that periodontitis affected 42% of adult Americans, with 7.8% of cases being severe. [7] This poll verified that about 50% of adults in the US (30 years of age or older) suffer from periodontitis, which is highly prevalent in the country. 743 million people worldwide, or around 11% of the total population, may suffer from severe periodontitis (Figure 1). [8–14]

Fig. 1 – The frequency of severe periodontitis worldwide8?10 in comparison to diabetes11, hypertension12, depression13 and asthma.14
PERIODONTAL DISEASE:
The term "periodontal disease" refers to a wide spectrum of chronic inflammatory conditions that impact the connective tissue—the collagen fibres in the connective tissue that connect a tooth to the alveolar bone—as well as the bone, ligament, and gingiva, the soft tissue that surrounds teeth. The first indication of periodontal disease is gingivitis, a localised inflammation of the gingiva brought on by bacteria in dental plaque, a microbial biofilm that develops on the gingiva and teeth (FIG. 2). Gingivitis that is left untreated can progress to chronic periodontitis, which is characterised by deep periodontal "pockets" can result in tooth loss. Gingiva, bone, and ligament loss are the conditions causes. By merely adding to conditions like diabetes mellitus and atherosclerosis, periodontal disease may make them worse to the body's overall inflammatory burden.[15-17]
Gingivitis:
People who forgo brushing their teeth for ten to twenty days are more likely to develop chronic gingivitis. [18] In those experiencing a hormonal disruption, such as youngsters going through puberty or pregnant women, the clinical symptoms are more severe and the gingiva is more edematous and irritated. About 30% of people on certain pharmacological regimens, including phenytoin (used to treat epilepsy), cyclosporine (an immunosuppressive medication), and nifedipine (a calcium channel blocker used in hypertensive patients), can develop gingival overgrowth. The increased response to microbial plaque that is required for gingivitis and, as a result, the drug's effects on the gingiva is known as gingival overgrowth. Also, smoking appears to diminish gingival inflammation. This could be because nicotine constricts blood vessels, which reduces tissue edoema.
It can be reversed at the first stage by keeping good oral hygiene, but if plaque is not eliminated at this point, tartar or calculus will form and will not be removed with a toothbrush or floss. Tartar causes germs to target deeper tissues, weakening the periodontal ligaments around teeth and causing alveolar bone resorption. [19]The term "periodontital pocket" refers to the space that develops between the gingiva and the tooth; periodontitis or periodontal disease is the major term for this illness. The development of microbial plaque determines how severe this illness is. [20]
There are numerous tests available to diagnose periodontal disease, including tissue engineering, laser therapy, haematological screening, and radiography. Depending on the disease's chronology, a variety of therapeutic options—both surgical and non-surgical—are available to slow the disease's advancement. Maintaining proper dental hygiene and providing intensive care are the two main ways in which this disease is maintained. [21, 22]

Figure 2 | Healthy and Diseased periodontium.
(a) Healthy periodontal tissues.
(b) Early gingival inflammation (c) Clinical appearance of chronic periodontitis.
2. Stages:
There are mainly four stages in periodontal diseases which includes different clinical sign & symptoms and radiological screening as given below:[ 23–25]

Periodontitis can only be reversed at this point. Plaque starts to build around teeth at this point. At this point, the most common painless symptoms include bad breath, reddish-swollen gums, and bleeding when you brush and floss. Regular exams and Keeping your teeth clean can help reverse it.. Typically, there is a clinical attachment loss of 1-2 mm, Under fifteen percent of the bone around the root has been lost, and a 4 mm probe depth is required.
It is the second stage of periodontal disease's. While not reversible, it is tolerable with good dental hygiene. At this point, the infection begins to spread and weaken the surrounding tissues. the patient has bleeding when brushing or flossing, severe bad breath, and noticeable gum irritation. The space between teeth also steadily widens. This results in a clinical bonding loss of 3–4 mm, less than 15–33% of bone loss surrounding the root, and a probing depth of 5 mm or less.
This stage cannot be reverted, just like second stage. The same symptoms as the moderate stage are present, but there is more space between teeth and gum recession. At this point, procedures including scaling, flap operations, and extensive cleaning can be performed. clinical bonding loss of five millimetres or more, 33% of tooth loss involving four teeth or less, and complex problems such Class II–III furcations, substantial ridge abnormalities, and/or probing depths of six millimetres or more.
The final stage of periodontal disease is characterised by 50–90% loss of periodontal tissues as well as additional symptoms like pus-filled, swollen gums, teeth that are loose, intense halitosis, and cold sensitivity. Not addressing it can lead to gum recession, greater voids or spaces between teeth and gums, patient needling dentures, and other potentially worse health conditions.. Treatment for periodontitis can include routine examinations, cleanings, and upholding proper dental hygiene. Less than 20 teeth (10 opposing pairs) remain after secondary occlusal trauma, significant ridge abnormalities, bite collapse, and pathologic migration of teeth.
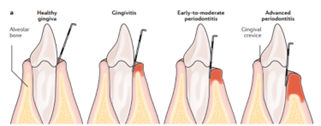
FIG 3: The principal phases of gum disease.
3. Types of Periodontitis : [26–29]
3.1. Gingivitis:
Gingivitis, as previously mentioned, is gum tissue inflammation and is curable with proper oral hygiene.
3.2. Chronic periodontitis:
This kind of periodontal disease can cause severe bad breath, bleeding during flossing or brushing, and persistent gum irritation. loss of ligaments, bone, and epithelium that cannot be reversed.
3.3. Aggressive periodontitis
Both localised and generalised variants of this early-onset persistent inflammatory periodontal disease often appear between puberty and the beginning of the the third decade of existence. The same symptoms as chronic periodontitis are present.
3.4. Necrotizing ulcerative gingivitis:
People with HIV, immune suppressants, and malnutrition are the main populations affected. Necrosis is the death of a live tissue or cell. It typically happens results in lack of nutrition, which is necessary for people to stay healthy.
3.4.1. Peri-implant mucositis:
It is linked to soft tissue inflammation around dental implants without any evidence of bone loss. Symptoms include bleeding when brushing and red or sore gums surrounding implants.
3.5. Systemic chronic periodontitis:
Patients with systemic condition are susceptible to this kind of persistent periodontal disease. Systemic diseases including diabetes millitus , coronary heart disease, respiratory disorders, etc. can cause gum inflammation.
4. Microbiology of periodontitis disease:
There are about 300–400 different types of bacteria present in human subgingival plaque samples. Ten to twenty of those species might be involved in the pathophysiology of destructive periodontal disease. [30] For these bacteria to live and harm the periodontal tissues, they must be able to colonise.
Microbes need to be able to colonise subgingival locations in order to
1) adhere to the tissues of the periodontal region,
2) multiply,
3) rival other microorganisms in their environment and
4) defend themselves from host defence mechanisms.
Certain components found in gingival crevicular fluid and saliva can even precipitate and kill germs directly. Other host processes must be overcome if a bacterium manages to evade the salivary inhibitory factors and adheres to the subgingival area's surface.
These include the phagocytic death of bacteria by neutrophils, the sloughing off of epithelial cells if bacteria have attached to an epithelial surface, and antibodies that prevent bacteria from adhering to epithelial surfaces. A species comes into contact with a variety of host immune cells, including neutrophils, macrophages, B and T lymphocytes, and lymphocytes, once it has broken through the underlying connective tissue. To thrive, bacteria must be able to evade each among these systems for host response.
Gram-negative anaerobic bacilli, together with a small number of anaerobic cocci and a significant number of anaerobic spirochetes, are the predominant bacteria responsible for periodontal disease Porphyromonas gingivalis, Treponema denticola, Bacteroides forsythus, Prevotella intermedia, and Actinobacillus actinomycetemcomitans are the primary microorganisms associated has extensive, harmful periodontal lesions. [31] Compared to individuals in good health, those with gingivitis or edentulous subjects, P. gingivalis is more commonly found in cases of severe adult periodontitis, destructive forms of the disease, and active lesions. They are seen in locations where the disease has recurred following therapy, but their numbers are decreased at areas where treatment has been successful. [32, 33, 34]
In contrast to healthy sites or gingivitis, B. forsythus has been detected in greater quantities in areas with devastating periodontal disease. [35] According to Genco et al. [36] periodontitis appears to be caused by more uncommon or external anaerobic microbes, whereas gingivitis is mostly caused by organisms of the normal flora.
5. Pathophysiology:
Dental plaque is the primary cause of gingivitis and periodontitis. Approximately 150 distinct species of bacteria have been recognised in a single human, while 700 different types microorganisms have been found in dental calculus. The species include viruses, spirochetes, and Gram-negative anaerobic bacteria. The "pathogenic unit" in cases of chronic periodontal disease is formed by an imbalance between these bacteria. [37]
The cause of gingivitis is microbial biofilm. Microbial biofilm development is reliant on dysbiotic ecological shifts in harmful byproducts and an enzyme that breaks down periodontal tissue. Microbial biofilms, which adhere to one another on the surface of teeth, are a type of matrix fixed with various microbial species colonies. [38] The following are the seven phases of plaque biofilm formation:
- Pellicle formation
- Transport
- Long range interactions
- Interactions at short range
- Co-aggregation
- Multiplications
- Detachments
The aetiology of periodontal disease is not solely attributed to microbial films; the immune system of the host cell also plays a role in the breakdown of periodontal ligaments. [39]A notable variation in dental plaque and the host immunity system arises from the loss of the equilibrium between the host and the microbial biofilm cells. This leads to an increase in inflammatory cells, which in turn causes the deterioration of bone and periodontal tissue. Forty Consequently, the continuous endurance of microbiological biofilms causes a decrease in anti-inflammatory cells, including granulocytes, lymphocytes, and neutrophils which exacerbates alveolar bone loss caused by osteoclasts and causes ligament fibre breakdown that eventually ends in chronic periodontitis. [41]
6. Risk factor causing periodontitis
When it comes to periodontal disease, there are two different types of risk factors: one that can be changed, and the other that cannot. [42]
- Smoking: Need an additional incentive to give up smoking? One of the biggest risk factors for the emergence of gum disease is smoking. Furthermore, smoking can make treatment less likely to be successful.
- Hormonal shifts in women and girls: These alterations may increase gum sensitivity and facilitate the development of gingivitis.
- Diabetes: Diabetes increases a person's susceptibility to infections, particularly gum disease.
- Other Illnesses and their treatments: Gum health can be adversely affected by cancer treatments as well as diseases like AIDS and its associated therapies.

FIG 4: Risk factors

Table No 1 : modifiable and non- modifiable factors
Medications: Numerous over-the-counter and prescription drugs are available that can decrease saliva production, which has a mouth-protecting effect. The mouth becomes susceptible to illnesses like gum disease when there is insufficient saliva. Furthermore, several medications possess the capacity to induce aberrant gum tissue growth, which can make it challenging to maintain clean teeth and gums.
7. Treatment and Management:
There are three phases for treatment of periodontal disease:
-
- Initial therapy or non – surgical treatment
- Corrective therapy.
- Supportive therapy
Initial therapy: This treatment is administered during the initial phases of gum disease in order to identify any modifiable risk factors and limit the growth of microbial plaque. [43] Doctors advise patients on proper oral hygiene practices, quitting behaviours such as smoking, drinking alcohol, and chewing pan masala. They also provide instructions on the sort of toothbrush to use, how to utilise interdental appliances, dentifrices, mouthwash, etc. [44] If diabetes mellitus or other periodontal risk factors are found, the patient should be counselled appropriately. Since six weeks is the minimal amount of time needed for tissue or periodontal ligament repair, the therapy is reevaluated after eight to twelve weeks. The following therapies are part of the initial treatment:
7.1.1. Tooth brushing:
To eliminate dental plaque, toothbrushes with manual or electronic functions are offered. A 2005 study by Robinson et al. found that powered toothbrushes that rotate and oscillate are more effective at eliminating dental plaque. [45]
7.1.2. Interdental cleaning:
To effectively remove dental plaque, A good toothbrush can only clean around 65% of the tooth surface. Therefore, Additionally, interdental cleaning required to remove microbial biofilms from items like tape, motorised flossers, and dental floss. Dental floss and tape are recommended to patients when the interdental papillae are fully emaciated, as this improves the clinical results of periodontal disease. [46]
7.1.3. Adjunctive pharmacological agent:
To boost the effectiveness of mouthwashes and toothpaste, numerous pharmaceutical ingredients have been introduced. A common medication like as gluconidine chlorhexidine is regarded as the gold standard for treating gingivitis and plaque. [47] It is mostly added to toothpaste, gel, and mouthwash.
There are several instances of supplemental

Table no 2: pharmacological agent
-
- Non-surgical treatment: [48,49]
Treatment for gingivitis possibly less combative in the early stages and include the following:
- Scaling: Scaling aids in the removal of microbial biofilms and calculus from the gums. It can be controlled by an ultrasonic device or by hand devices.
- Root planning: Root planning prevents more tartar accumulation while also smoothing the root surface. Additionally, it eliminates harmful byproducts to lessen inflammation and promote healing of the gums' adhesion to the tooth surface.
- Antibiotics: Antibiotics administered topically or orally are used to prevent the growth of microbial biofilms. Topical antibiotics are injected into periodontal pockets or the gingival sulcus by gels, implants, etc. On the other hand, oral antibiotics remove bacterial infections from the surfaces of teeth and gums.
- Corrective therapy : [50–53]
There are numerous surgical options available to address periodontal disease. (figure 5)

7.3.1. Supportive therapy:
This treatment plan is recommended to maintain periodontal health and avoid disease recurrence. [54] As part of this therapy, the patient will have routine check-ups to assess their periodontal condition and to retrain them in maintaining proper oral hygiene and plaque control. [55]
8. Drug delivery system:
A drug delivery system (DDS) is characterized as a formulation or a device that regulates the pace, time, and location of drug release in the body, thereby facilitating the entry of a medicinal material and enhancing its safety and efficacy. The delivery of the medicinal product, the product's release of the active chemicals, and the subsequent movement of the active ingredients across biological membranes to the site of action are all included in this process.[56]
The introduction of medications in human body may be accomplished by various anatomic pathways. [57] Finding the best possible administration route is essential for achieving the desired therapeutic outcome. As a result, while delivering a medication, many elements need to be taken into account, including the drug's own features, the illness that has to be treated, and the intended therapeutic duration. Medications might be administered systemically or straight to the intended organ or tissue. [58]
Drug Delivery Routes:
The human body can absorb drugs through a variety of anatomical pathways. They may be directed against specific organs and illnesses, or they may be meant to have systemic effects. The illness, the desired outcome, and the substance that is available all influence the route of administration selection. Medication might be injected systemically and directed towards the diseased organ, or it can be delivered directly to the organ that is ill.[56]
As previously mentioned, the primary goal of employing a DDS is to maintain the drug's level in the body inside the therapeutic window in addition to delivering a biologically active chemical in a regulated manner (time period and releasing rate). Additionally, the medication can be directed towards a particular tissue or organ (targeted drug delivery). [59]Drug carriers, often polymers (biopolymers or synthetic polymers) whose characteristics could be adjusted to increase DDS efficiency, were used to address the first two features.
Local drug delivery system:
Goodson of the Dental Research Centre in Forsyth was the pioneer in developing the therapy approach. This therapy works well because it gets to the root of the periodontal pocket and keeps the antibacterial concentration high enough for the desired amount of time. The periodontal pocket offers an easily accessible natural reservoir for the implantation of a delivery device, which is submerged in gingival crevicular fluid.[60]
Fibres, films, injectable systems, gels, strips, and compacts, vesicular systems, microparticle systems, and nanoparticle (NP) systems are among the forms in which different formulations are offered. [60]
The commonly used antimicrobial delivery systems are:
• Tetracycline Fiber
• Metronidazole gel
• Chlorhexidine chip
• Minocycline gel
• Doxycycline polymer

Fig 6: Various Drug Delivery System
9. Herbal medications in periodontal therapy:
For thousands of years, medicinal plants have been utilized as traditional remedies for a wide range of human ailments in many different parts of the world. They still serve as the main source of medication in developing nations' rural areas. Approximately 80% of the population in underdeveloped nations receives their medical care from traditional practitioners. A wealth of physiologically active molecules exist in the natural products made from medicinal plants, many of which have served as the foundation for the creation of novel lead chemicals for the pharmaceutical industry. The growing resistance of many common pathogens to commonly employed therapeutic drugs, such as antibiotics and antiviral medicines, with regard to diseases caused by microbes has sparked fresh interest within the recognition of innovative in anti-infective compounds. With only 1% of the 500,000 plant species that exist worldwide having undergone phytochemical research, there is a lot of room for the finding of novel bioactive chemicals. [61]
Some of the medicinal plants which are used in treatment of periodontitis are mentioned below:
The most popular spice in homes is turmeric, which possesses antibacterial and anti-inflammatory properties because of the existence of curcumin. Turmeric is an easy way to get rid of gum disease-causing germs and reduce inflammation and pain. Moreover, it enhances dental health. Both turmeric and chlorhexidine gluconate mouthwash can be used as an effective adjunct to mechanical plaque control methods in the prevention of plaque and gingivitis, according to a study titled "Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: a clinical and microbiological study" [62] One could argue that turmeric is a useful supplement to mechanical plaque management. To prove mouthwash with turmeric as an inexpensive way to prevent plaque, Further investigation is required.
Since ancient times, triphala has been a well-known powdered substance in ayurvedic treatment. It is composed of equal portions of Bahera (Terminalia belerica), Haritaki (Terminalia chebula), and Amalaki (Emblica officinalis). Strong antibacterial, antioxidant, and anti-collagenase qualities are present in triphala.[63] The antioxidants in triphala lessen the load of oxidative stress and shield cells from harm from free radicals. The most potent antioxidant is bahera, which is followed by haritaki and amalaki. Triphala mouthwash has been demonstrated in a clinical experiment to have antiplaque and anti-inflammatory properties comparable to 0.2% chlorhexidine.[64]
Because cinnamon extract can lower plaque and gingivitis, an investigation into the effects of cinnamon extract on gingival health suggested that cinnamon might be a useful agent. [65] Sumac (Rhus coriaria) is a well-known spice with anti-inflammatory, antibacterial, and antioxidant qualities that make it a popular choice in herbal medicine. [66] Sumac extracts may be able to decrease the loss of alveolar bone in rats with periodontal disease via influencing the overall levels of oxidative stress.[67]
Green tea, widely recognised for its potential to support weight loss, is derived from Camellia sinensis leaves that have seen just a slight degree of oxidation throughout the processing process. Strong effects on periodontal infections have been noted for green tea catechins. Porphyromonas gingivalis and Prevotella spp. are examples of anaerobic bacteria that are the primary cause of periodontitis. According to in vitro research, these substances stop P. gingivalis, Prevotella intermedia, and Prevotella nigrescens from growing.[68,69,70] Additionally, it stops P. gingivalis from adhering to human buccal epithelial cells. [71]
However, further studies are required to use this herbal product as a novel therapeutic approach to treat bone degenerative disorders such as periodontitis, rheumatoid arthritis, and osteoporosis. [72]
The usage of aloe vera has been supported by numerous research because it helps reduce gingival irritation. It eliminates the germs that causes gingivitis. It also aids in hastening the healing process. The benefits of massaging aloe gel into the gums are numerous. [73]
Another of the best remedies for gingivitis and other gum-related conditions is clove oil. Gingivitis can be relieved simply by chewing a clove or applying clove oil to the gums. [74]
Guavas are high in vitamin C, they are also thought to be a great periodontitis treatment. It functions as an anti-plaque agent, aiding in the removal of plaque that has accumulated [75] on the gingiva and teeth. Its analgesic and anti-inflammatory qualities aid in lowering pain and swelling on the gingiva .
Despite its sweetness, honey's antibacterial qualities are good for the periodontium. It offers the nutrients required to keep gums healthy and is also high in vitamins and minerals. Monocyte activity was found to be impacted by exposure to a honey solution in vitro. Mouthwashes containing propolis, which is found in bee products, were discovered to have antimicrobial activity against Streptococcus mutans. [76]As a result, they can be used as an alternative treatment to prevent dental caries and to lessen the buildup of plaque and polysaccharide production. [77]
According to a recent report, gingivitis decreased by 95% in periodontal pockets irrigated with 10% propolis solution. This suggests, based on both clinical and microbiological parameters, that propolis extract subgingival irrigation is a more effective adjunct to periodontal treatment than scaling and root planning. [78] According to these findings, patients receiving orthodontic treatment may be able to avoid caries and gingivitis by applying or consuming honey topically.[79] These first findings will need to be confirmed by additional research.

FIG. 7 Various Drug Delivery System

Table: List of plants with medicinal value in dentistry.
10. Challenges with current periodontal disease management:
Surgery to eliminate periodontal pockets is very expensive. For example, the cost of surgery in the United States ranges between $4000-5000 and is not covered by health insurance. [82]
For periodontal disease, professional scaling, root planing, and tooth brushing are the usual non-surgical therapies. Strict plaque control with antibacterial mouthwashes (like the gold standard, chlorhexidine) and/or systemic use of sub-antimicrobial dose doxycycline as a new adjunct host-modulatory therapy option [87] as well as local and systemic administration of antibiotics (tetracycline and metronidazole, the latter of which primarily acts on anaerobic bacteria) are also recommended.
Non-surgical therapies have significant drawbacks. Mechanical plaque removal may lead to pathogen recolonization and is not always effective in addressing all bacterial hiding spots [83]. Chlorhexidine has a number of undesirable effects when taken for longer than 15 days, including darkening of the teeth and tongue, increased mouth sensitivity, and allergic responses [88,83]. It was also found that dental calculus accumulated more readily when chlorhexidine was used for an extended period of time [89].
10.1 Side Effects of Antibiotics describe in Periodontal Recipes:
The literature was searched for information on the adverse effects of various antibiotics that are most commonly administered in periodontal formulations. Preferably, percentages of the data were obtained. The prescription antibiotic is reflected in the order of these statistics.
- Penicillin: Upon application, amoxicillin causes hypersensitivity, manifesting as cross-reaction and sensitivity to breakdown products of alkaline hydrolysis. Penicillin anaphylactic shock occurs in 0.05% of cases, but penicillin allergic responses can develop in 5–8% of cases [90]. Common oral lesions can also be associated with other negative outcomes, including nephritis, eosinophilia, and hemolytic anaemia. Using an oral dosage can cause nausea, vomiting, diarrhoea, and gastrointestinal problems..
- Cephalosporins: Similar to penicillins, cephalosporins can make patients more sensitive to their surroundings. Cephalosporin hypersensitivity may not manifest in penicillin allergy sufferers due to their slightly different chemical structure. About 5–10% of instances result in allergic responses.
- Tetracyclines: Tetracycline causes diarrhoea, vomiting, and nausea as gastrointestinal side effects. Tetracycline alters the typical gut flora, promoting the overgrowth of Proteus, Clostridium, and Pseudomonas while suppressing coliform organisms. Tetracycline use is linked to vaginal candidiasis [91]. If tetracycline is administered during specific stages of pregnancy, such as the foetal development stage, it permanently alters the structure of the developing teeth. When tetracycline is administered intravenously or in patients with a history of hepatic failure, liver damage might result. When tetracycline is administered following nitrogen retention products and in combination with diuretics, kidney damage might ensue. Venous thrombosis is accompanied by local tissue damage and light sensitivity as side effects. In 35–70% of instances, there is nausea and dizziness [91].
- Metronidazole: Metronidazole, an antiprotozoal, has a stronger antibacterial action against anaerobes like Clostridium. The CSF fluid is penetrated by the oral dose of 250 mg. The liver is where metronidazole is metabolised. Metronidazole is an effective treatment for bacterial vaginosis. The most common adverse effects are neutropenia, stomatitis, diarrhoea, and nausea. Metronidazole provides dentists with a number of benefits, a high degree of efficiency, and comparatively few adverse effects. Suspects are still developing a clinical resistance to this particular antibiotic [92]. Tetracycline appears to induce tooth discolouration, gastrointestinal issues, uncommon allergies, photosensitivity, nitrogen retention, and increasing uremia, according to research presented in the literature. Interactions with coumarin are evident.
- Carbapenem: The adverse effects of carbapenem include: gastrointestinal disturbances, vomiting, nausea, diarrhoea, and haematological difficulties in 0.3% of cases; pseudomembranous colitis (0.16%); skin allergy development in 2.7% of cases; and nervous system abnormalities in 3% of cases. When analysing the indications for antibiotic medication, side effects are calculated, with indications pertaining to prophylaxis or therapy. Antibiotics are foreign substances that the body absorbs, which results in side effects. Their chemical compositions have the potential to be harmful. By means of the IgE, they can induce allergies. The biological impacts are also manifested in the body as foreign material
The results derived from the aforementioned data regarding the adverse effects of antibiotics used in the treatment of periodontal diseases indicate that patients are at risk of:
- Allergy (5.5%),
- Nephritis (4%),
- Hematological problems (2–3%),
- Gastrointestinal problems (5%),
- Disturbance in the nervous system (3%),
- Signs of allergy on the skin (5.8%),
- Problems with electrolytes displayed in lower percentage
11. Herbal medicine as an alternative solution for management of periodontal disease:
Safe and effective alternatives to current treatment methods are needed due to the danger of antibiotic resistance and their shortcomings. [83, 85, 93, 94, 95] Much in vitro and in vivo research has been conducted in the past ten years to determine whether medicinal plants with well-established antibacterial and anti-inflammatory properties are effective in treating periodontal disease. In order to ascertain whether herbal medicine can help cure and control periodontal disease, this research examines the available data.
Benefits of herbal drugs:
Natural remedies have been used for a very long time and are more well-liked by the general public and patients. The only reliable source of affordable medications for the world's expanding population is herbal medicine because it is a renewable resource. Medicinal plant availability is not a problem, particularly in emerging nations with significant agroclimatic, cultural, and ethnic biodiversity like India. It is environmentally friendly to raise and process therapeutic plants and herbal products. Herbal medicine has contributed a great number of beneficial and diverse medications to modern medicine worldwide.[96]
Comparing plant materials therapy to traditional agents like chlorhexidine, it generally offers a better safety profile and fewer adverse effects. It is critical to recognise when adverse effects could lead to therapeutic discontinuation, particularly in cases of polypharmacy and chronic conditions. The effects of periodontitis include tooth loss and associated diseases in addition to other factors that make them more likely to occur, have a more severe course, or require more intensive treatment. Diabetes and periodontitis are known to be related. Diabetes patients with periodontitis experience a more severe course of the disease, greater degradation of periodontal tissues, and worse glycemic control when they have periodontal diseases. [97] Additionally, periodontitis increases the risk of cardiovascular illnesses. Increased arterial stiffness is seen, which is a sign of increased risk for cardiovascular disease.[98] Furthermore, those with periodontitis have a twofold increased risk of thrombotic and cardiovascular stroke. [99] Thus, it is imperative that periodontitis be prevented and managed safely and effectively starting in the early phases of development.
As of right now, herbal components show promise as a therapy alternative adjuvant to traditional management techniques like scaling, which can improve outcomes and lessen the burden of aggressive medicines. Plant-based raw materials have demonstrated encouraging activity in preventing periodontitis. [100] but more study is need to assess their efficacy and long-term safety.
Apart from their biocompatibility and plant-based friendliness, biomaterials typically have a cheaper cost and a superior safety profile. Furthermore, there is strong evidence that biomaterials can be utilised as an adjuvant or replacement for traditional medicines, hence boosting their efficacy.
CONCLUSION
Dental and oral health are closely tied to overall well-being and are some of the most widely used health concerns today. Periodontitis, a widespread issue, encompasses a range of chronic inflammations that impact the connective tissue around teeth. One of the major challenges in managing periodontal disease is the increasing risk of antibiotic and antimicrobial resistance. Consequently, there is a global demand for alternative prevention and treatment options that are safe, effective, and affordable, especially in developing countries. This need is driven by the rise in disease incidence, increased bacterial resistance to existing antibiotics and chemotherapeutics, opportunistic infections in immunocompromised individuals, and financial constraints. Current commercial agents, although available, can disrupt oral microbiota and cause side effects such as vomiting, diarrhea, and tooth staining. Documented bacterial resistance to commonly used oral infection antibiotics and toxicity issues with other antibacterial agents like cetylpyridinium chloride, chlorhexidine, amine fluorides, and ethanol (in mouthwashes linked to oral cancer) underscore the need for alternatives.
Natural phytochemicals from plants used in traditional medicine are emerging as viable substitutes for synthetic chemicals. Herbal medicines, known since ancient times for their Antimicrobial, Antioxidant and Anti-inflammatory properties, help reduce alveolar bone loss characteristic of periodontitis. Their efficacy, safety, accessibility, and controlled treatment potential make herbal therapies suitable for dentistry, mirroring their use in other medical disorders. Although many studies highlight the effectiveness of herbal medicines as alternatives to conventional therapies, more research is needed on their clinical applications in periodontics. This systematic review suggests that oral Herbal care products could significantly enhance periodontal treatment outcomes. Plant-derived actives, used as adjuncts, offer substantial clinical benefits without any adverse effects and are suitable as substitutes. Future research ought to adhere to established procedures to better understand the effects of various herbal dental products.
REFERENCE
- Beard, J.R.; Bloom, D.E. Towards a comprehensive public health response to population ageing. Lancet 2015, 385, 658–661.
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858.
- Petersen, P.E.; Yamamoto, T. Improving the oral health of older people: The approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2005, 33, 81–92.
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597.
- WHO. Europe Disease Prevention—Data and Statistics. 2018. Available online: https://www.euro.who.int/en/health-topics/ disease-prevention/oral-health/data-and-statistics (accessed on 9 December 2020).
- Prasai Dixit, L.; Shakya, A.; Shrestha, M.; Shrestha, A. Dental caries prevalence, oral health knowledge and practice among indigenous Chepang school children of Nepal. BMC Oral Health 2013, 13, 1–5.
- Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009?2014. J Am Dent Assoc 2018;149 576?588.e6.
- Richards D. Review finds that severe periodontitis affects 11% of the world population. Evid Based Dent 2014;15:70–1.
- Kassebaum NJ, Bernabe E, Dahiya M, et al. Global burden of severe periodontitis in 1990?2010: a systematic review and meta-regression. J Dent Res 2014;93:1045–53.
- Frencken JE, Sharma P, Stenhouse L, et al. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol 2017;44(Suppl 18):S94–S105.
- International Diabetes Federation. Global diabetes data report 2010?2045. Available from: https://diabetesatlas.org/ data/. Accessed May 9, 2020. Fig. 10 – A decision tree for treating a patient with periodontitis. 472 kwon et al.
- World Health Organization. WHO fact sheet on hypertension. Available from: https://www.who.int/news-room/factsheets/detail/hypertension. Accessed May 9, 2020.
- World Health Organization. WHO fact sheet on depression. Available from: https://www.who.int/news-room/factsheets/detail/depression. Accessed May 9, 2020.
- World Health Organization. WHO factsheet on asthma. Available from: https://www.who.int/news-room/factsheets/detail/asthma. Accessed May 9, 2020
- Gotsman, I. et al. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J. Periodontol. 78, 849–858 (2007).
- Jeffcoat, M. K. et al. Periodontal disease and preterm birth: results of a pilot intervention study. J. Periodontol. 74, 1214–1218 (2003).
- Khader, Y. S., Dauod, A. S., El Qaderi, S. S., Alkafajei, A. & Batayha, W. Q. Periodontal status of diabetics compared with nondiabetics: a metaanalysis. J. Diabetes Complications 20, 59–68 (2006)
- Lo¨e H, Theilade E, Jensen S. Experimental gingivitis in man. J Periodontol 1965: 36: 177–187 Lo¨e H, Theilade E, Jensen S. Experimental gingivitis in man. J Periodontol 1965: 36: 177–187
- Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, et al. Periodontal Disease and NIDDM in Pima Indians. Diabetes Care. 1990;13(8):836–40.
- Holand C. Rethinking perio classification for the 21st century. BDJ Team. 2019;6(3):24–7.
- Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36(6):458–67.
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;89(1):1–8.
- Fine DH, Patil AG, Loos BG. Classification and diagnosis of aggressive periodontitis. J Periodontol. 2018;89:S103–7.
- Dietrich T, Ower P, Tank M, West NX, Walter C, Needleman I, et al. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions – implementation in clinical practice. Br Dent J. 2019;226(1):16–22.
- Graetz C, Mann L, Krois J, Sälzer S, Kahl M, Springer C. Comparison of periodontitis patients’ classification in the 2018 versus 1999 classification. J Clin Periodontol. 2019;46(9):908–17
- Dietrich T, Ower P, Tank M, West NX, Walter C, Needleman I, et al. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions – implementation in clinical practice. Br Dent J. 2019;226(1):16–22.
- Herrera D, Retamal-Valdes B, Alonso B, Feres M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol. 2018;89(1):S85–S102.
- Gher M, Vermino AR. Root morphology- Clinical signifance in pathogenesis and treatment of dental plaque gingivitis. J Am Dent Assoc. 1990;12:36–41.
- Tonetti MS, Sanz M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J Clin Periodontol. 2019;46(4):398–405.
- Socransky S, Haffajee A. Evidence of bacterial aetiology: a historical perspective. Periodontol 2000 1994: 5: 7–25.
- Zambon JJ. Periodontal diseases: microbial factors. Ann Periodontol 1996: 1: 879–925.
- Bragd L, Dahle´n G, Wikström M, Slots J. The capability of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis, Bacteroides intermedius to indicate progressive periodontitis; a retrospective study. J Clin Periodontol 1987: 14: 95–99
- Haffajee A, Dzink J, Socransky S. Effect of modified Widman flap surgery and systemic tetracycline on the subgingival microbiota of periodontal lesions. J Clin Periodontol 1988: 15: 255–262.
- van Winkelhoff AJ, van der Velden U, de Graaff J. Microbial succession in recolonizing deep periodontal pockets after single course of supra- and subgingival debridement. J Clin Periodontol 1988: 15: 116–122.
- Lai C-H, Listgarten M, Shirakawa M, Slots J. Bacteroides forsythus in adult gingivitis and periodontitis. Oral Microbiol Immunol 1987: 2: 152–157.
- Genco R, Zambon J, Christersson L. The origin of periodontal infections. Adv Dent Res 1988: 2: 245–259.
- Kato T, Fujiwara N, Kuraji R, Numabe Y. Relationship between periodontal parameters and non-vital pulp in dental clinic patients: a cross-sectional study. BMC Oral Health. 2020;20(1):125–53
- Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32(s6):87–107.
- Jepsen S, Suvan J, Deschner J. The association of Periodontal diseases with metabolic syndrome and obesity. Periodontol. 2000;83(1):3–12.
- Ji S, Choi Y. Microbial and Host Factors That Affect Bacterial Invasion of the Gingiva. J Dent Res. 2020;12:19–62.
- Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162(2):22–38.
- Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol. 2000;62(1):1049–52
- Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontol. 2000;58(1):37–68.
- Nibali L, D’Aiuto F, Donos N, Griffiths GS, Parkar M, Tonetti MS, et al. Association between periodontitis and common variants in the promoter of the interleukin-6 gene. Cytokine. 2009;45(1):50–4.
- Hine MK. The use of the toothbrush in the treatment of periodontitis. J Am Dent Assoc. 1950;41(2):158–68.
- Erbe C, Klees V, Braunbeck F, Ferrari-Peron P, Ccahuana-Vasquez RA, Timm H, et al. Comparative assessment of plaque removal and motivation between a manual toothbrush and an interactive power toothbrush in adolescents with fixed orthodontic appliances: A singlecenter, examiner-blind randomized controlled trial. Am J Orthod Dentofac Orthop. 2019;155(4):462–72.
- Efstratiou M, Papaioannou W, Nakou M, Ktenas E, Vrotsos IA, Panis V. Contamination of a toothbrush with antibacterial properties by oral microorganisms. J Dent. 2007;35(4):331–7.
- Sanz I, Alonso B, Carasol M, Herrera D, Sanz M. J Evid Based Dent Pract. 2012;47(3):18–26.
- Cleland WP. Nonsurgical periodontal therapy. Clin Tech Small Anim Pract. 2000;15(4):221–5.
- Friedman S, Abitbol S, Lawrence H. Treatment Outcome in Endodontics: The Toronto Study. Phase 1: Initial Treatment. J Endod. 2003;29(12):787–93.
- Jain N, Jain GK, Javed S, Iqbal Z, Talegaonkar S, Ahmad FJ, et al. Recent approaches for the treatment of periodontitis. Drug Discovery.
- Cobb CM. Lasers and the treatment of periodontitis: the essence and the noise. Periodontology. 2000;75(1):205–295.
- Nowzari H. Aesthetic osseous surgery in the treatment of periodontitis. Periodontol 2000. 2001;27(1):8–28.
- Rohlin M, Susanna A, Ekman A, Klinge B, Larsson G. Chronic Periodontitis -Prevention, Diagnos and Treatment - A systematic review. SBU Syst Rev Summ. 2004;31:239–52.
- Durham J, Fraser HM, McCracken GI, Stone KM, John MT, Preshaw PM. Impact of periodontitis on oral health-related quality of life. J Dent. 2013;41(4):370–6.
- Jain, K.K. (2008). Drug Delivery Systems - An Overview. In: Jain, K.K. (eds) Drug Delivery Systems. Methods in Molecular Biology™, vol 437. Humana Press. https://doi.org/10.1007/978-1-59745-210-6_1
- Zempsky WT. Alternative routes of drug administration—advantages and disadvantages (subject review). Pediatrics. 1998;101: 730–1.
- Golan DE, Armen H, Tashjian J, Armstrong EJ, et al. Principles of pharmacology: the pathophysiological basis of drug therapy. Philadelphia: Lippincott Williams&Wilkins; 2008.
- Bajpai AK, Shukla SK, Bhanu S, et al. Responsive polymers in controlled drug delivery. Prog Polym Sci. 2008;33:1088–118.
- Kaplish V, Walia MK, Kumar HS. Local drug delivery systems in the treatment of periodontitis: A review. Pharmacophore 2013;4(2):39-49.
- Bansal S et al. Mechanical Chemical and Herbal aspects of periodontitis : A Review . Int J Perio Res Dent, 2012; Vol 3 (5) : 1260 – 1267.
- Waghmare PF, Chaudhari AU, Karhadkar VM, Jamkhande AS. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaqueformation and gingivitis: a clinical and microbiological study. J Contemp Dent Pract. 2011 Jul 1;12(4):221-4
- Vani T, Rajani M, Sarkar S, Shishoo CJ. Antioxidant properties of the ayurvedic formulation triphala and its constituents. Pharm Biol 1997;35:313-7.
- Naiktari RS, Gaonkar P, Gurav AN, Khiste SV. A randomized clinical trial to evaluate and compare the efficacy of triphala mouthwash with 0.2% chlorhexidine in hospitalized patients with periodontal diseases. J Periodontal Implant Sci 2014;44:134-40.
- Gupta D, Jain A. Effect of Cinnamon Extract and Chlorhexidine Gluconate (0.2%) on the Clinical Level of Dental Plaque and Gingival Health: A 4-Week, Triple-Blind Randomized Controlled Trial.J Int Acad Periodontol. 2015:Jul;17(3):91-8.
- Rayne S, Mazza G. Biological activities of extracts from sumac (Rhus spp.): A review. Plant Foods Hum Nutr 2007;62:165-75.
- Saglam M, Köseoglu S, Hatipoglu M, Esen HH, Köksal E. Effect of sumac extract on serum oxidative status, RANKL/OPG system and alveolar bone loss in experimental periodontitis in rats. J Appl Oral Sci 2015;23:33-41.
- Asahi Y, Noiri Y, Miura J, Maezono H, Yamaguchi M, Yamamoto R, et al. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J ApplMicrobiol 2014;116:1164-71.
- Araghizadeh A, Kohanteb J, Fani MM. Inhibitory activity of green tea (Camellia sinensis) extract on some clinically isolated cariogenic and periodontopathic bacteria. Med Princ Pract 2013;22:368-72.
- Okamoto M, Leung KP, Ansai T, Sugimoto A, Maeda N. Inhibitory effects of green tea catechins on protein tyrosine phosphatase in Prevotella intermedia. Oral Microbiol Immunol 2003;18:192-5.
- Sakanaka S, Aizawa M, Kim M, Yamamoto T. Inhibitory effects of green tea polyphenols on growth and cellular adherence of an oral bacterium, Porphyromonas gingivalis. Biosci Biotechnol Biochem 1996;60:745-9
- Baek JM, Kim JY, Jung Y, Moon SH, Choi MK, Kim SH, et al. Mollugin from Rubea cordifolia suppresses receptor activator of nuclear factor- ?B ligand-induced osteoclastogenesis and bone resorbing activity in vitro and prevents lipopolysaccharide-induced bone loss in vivo. Phytomedicine 2015;22:27-35.
- Chhina S, Singh A, Menon I, Singh R, Sharma A, Aggarwal V. A randomized clinical study for comparative evaluation of Aloe Vera and 0.2% chlorhexidine gluconate mouthwash efficacy on de-novo plaque formation. J Int Soc Prev Community Dent. 2016 May-Jun;6(3):251-5
- Shivayogi Charantimath, Rakesh Oswal, Herbal Therapy in Dentistry: A Review, Innovative Journal of Medical and Health Science 1: 1 (2011) 1 – 4.
- Mittal P, Gupta V, Kaur G, Garg AK, Singh A. Phytochemistry and pharmacological activities of Psidium guajava: A review. Int J Pharm Sci Res 2010;1:9-19.
- Duailibe, S.A., Goncalves, A.G., Ahid, F.J., 2007. Effect of a propolis extract on Streptococcus mutans counts in vivo. J. Appl. Oral Sci.15, 420–423.
- do Amaral, R.C., Gomes, R.T., Rocha, W.M.S., Lemos, S., Abreu, R., Santos, V.R., 2006. Periodontitis treatment with Brazilian green propolis gel. Pharmacology (online), 336–341.
- Ahuja, V., Ahuja, A., 2011. Apitherapy––a sweet approach to dental diseases. Part II: propolis. J. Acad. Adv. Dent. Res. 2, 1–8.
- AL-Dany A Atwa et al ;Effect of honey in preventing gingivitis anddental caries in patients undergoing orthodontic treatment.The Saudi Dental Journal (2014) 26, 108–114
- Khare CP, editor. Indian Medicinal Plants. Berlin: Springer Verlag; 2007. p. 6 731
- Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 1999;86:985 90.
- Lazar, V., Saviuc, C., & Chifiriuc, M. C. (2016). Periodontitis and Periodontal Disease - Innovative Strategies for Reversing the Chronic Infectious and Inflammatory Condition by Natural Products. Current Pharmaceutical Design, 22(2), 230–237.
- Batista, A. L. A., Lins, R. D. A. U., de Souza Coelho, R., do Nascimento Barbosa, D., Moura Belém, N., & Alves Celestino, F. J. (2014). Clinical efficacy analysis of the mouth rinsing with pomegranate and chamomile plant extracts in the gingival bleeding reduction. Complementary Therapies in Clinical Practice, 20(1), 93–98. https://doi.org/10.1016/j.ctcp.2013.08.002.
- Kouidhi, B., Al Qurashi, Y. M. A., & Chaieb, K. (2015). Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microbial Pathogenesis, 80, 39–49. https://doi.org/10.1016/j.micpath.2015.02.007
- Karim, B., Bhaskar, D., Agali, C., Gupta, D., Gupta, R., Jain, A., Kanwar, A. (2014) ‘Effect of Aloe vera mouthwash on periodontal health: triple blind randomized control trial’, Journal of Oral Health and Dental Management, 13(1): pp. 14-19. PMID: 24603910
- Jain, N., Jain, G. K., Javed, S., Iqbal, Z., Talegaonkar, S., Ahmad, F. J., & Khar, R. K. (2008). Recent approaches for the treatment of periodontitis. Drug Discovery Today, 13(21–22), 932– 943. https://doi.org/10.1016/j.drudis.2008.07.010
- Preshaw, P. M. (2000). Host response modulation in periodontics. Periodontology, 48(2008), 92–110
- Balappanavar, A., Sardana, V. & Singh, M. (2013) Comparison of the effectiveness of 0.5% tea, 2% neem and 0.2% chlorhexidine randomized control trial. Indian Journal of Dental Research 24(1), pp. 26-34.
- Schwach-Abdellaoui, K., Vivien-Castioni, N., & Gurny, R. (2000). Local delivery of antimicrobial agents for the treatment of periodontal diseases. European Journal of Pharmaceutics and Biopharmaceutics, 50(1), 83–99. https://doi.org/10.1016/S0939- 6411(00)00086-2
- Bricker, S.L.; Langlais, R.P.; Miller, C.S. Oral Diagnosis, Oral Medicine and Treatment Planning, 2nd ed.; Hamilton, Ont.: London, UK, 2002; p. 854.
- Katzung, B.G.; Masters, S.B.; Trevor, A.J. Basic and Clinical Pharmacology, 12th ed.; Mc-Graw Hill Medical: Minneapolis, MN, USA, 2012.
- Ghayoumi, N. The use of metronidazole in the treatment of periodontal diseases. J. West. Soc. Periodontol. Periodontal Abstr. 2001, 49, 37–40. [PubMed]
- Srinath, J., & Lakshmi, T. (2014). Application of spices in dentistry - a literature review. International Journal of Drug Development and Research, 6(2), 1–9.
- Palombo, E. A. (Swinburne U. of T. (2011). Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-Based Complementary and Alternative Medicine, 2011, 15 pp. http://doi.org/10.1093/ecam/nep067
- Projan, S. J., & Youngman, P. J. (2002). Antimicrobials: new solutions badly needed. Current Opinion in Microbiology, 5, 463–465.
- Bansal S et al. Mechanical Chemical and Herbal aspects of periodontitis : A Review . Int J Perio Res Dent, 2012; Vol 3 (5) : 1260 – 1267.
- Llambés, F. Relationship between diabetes and periodontal infection. World J. Diabetes 2015, 6, 927.
- Schmitt, A.; Carra, M.C.; Boutouyrie, P.; Bouchard, P. Periodontitis and arterial stiffness: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 977–987.
- Sen, S.; Giamberardino, L.D.; Moss, K.; Morelli, T.; Rosamond, W.D.; Gottesman, R.F.; Beck, J.; Offenbacher, S. Periodontal Disease, Regular Dental Care Use, and Incident Ischemic Stroke. Stroke 2018, 49, 355–362.
- Salinas-Peña, E.A.; Mendoza-Rodríguez, M.; Velázquez-González, C.; Medina-Solis, C.E.; Pontigo-Loyola, A.P.; Márquez-Corona, M.; Rodríguez-Hernández, A.P.; Ximénez-Fyvie, L.A. Antibacterial properties in-vitro of Mexican serviceberry extracts against dental biofilm species. J. Berry Res. 2021, 11, 431–446.


 Saurabh R. Thadani*
Saurabh R. Thadani*
 saurabh Raghute
saurabh Raghute
 Bhoyar Samir
Bhoyar Samir











 10.5281/zenodo.12778872
10.5281/zenodo.12778872