Abstract
The ultimate goal of phototherapy based on nanoparticles, such as photothermal therapy (PTT) which generates heat and photodynamic therapy (PDT) which not only generates reactive oxygen species (ROS) but also induces a variety of anti-tumor immunity, is to kill tumors. In addition, due to strong efficacy in clinical treatment with minimal invasion and negligible side effects, it has received extensive attention and research in recent years. In this paper, the generations of nanomaterials in PTT and PDT are described separately. In clinical application, according to the different combination pathway of nanoparticles, it can be used to treat different diseases such as tumors, melanoma, rheumatoid and so on. In this paper, the mechanism of pathological treatment is described in detail in terms of inducing apoptosis of cancer cells by ROS produced by PDT, immunogenic cell death to provoke the maturation of dendritic cells, which in turn activate production of CD4+ T cells, CD8+T cells and memory T cells, as well as inhibiting heat shock protein (HSPs), STAT3 signal pathway and so on.
Keywords
pathological mechanism, nanoparticles, photothermal therapy, PTT, photodynamic therapy, PDT.
Introduction
Malignant tumors and their metastases have led to a high mortality rate in young people. The key is that many anti-tumor treatments, such as radiotherapy, chemotherapy, moleculartargeted therapy and immunotherapy, have too many systemic side effects; firstly, it causes severe damage to the immune system, and secondly, it also leads to long-term destruction of organ functions.1,2 As a result, few patients can be cured by clinical cancer treatment, and the disease has developed rapidly in recent years. Eventually, it can lead to serious consequences, such as multiple wasting death caused by organ failure, and severe malnutrition, etc.3 In recent years, PDT and PTT have been proposed to inactivate pathogens as new therapeutic regimens for tumor ablation and necrosis. PTT and PDT are mainly composed of near-infrared light (NIR) and nanoparticles, which, respectively, correspond to photosensitive (PS) and photothermal agents.
The application principle of PTT lies in the heat generated by gold nanoparticles and the activation of a photothermal agent under specific light wavelengths to kill cancer cells. The anti-tumor effect of PDT is under the interaction of the plasma nano-platform of the local electric field to produce single ROS and free radicals with cytotoxicity, short half-life and small diffusion rate, leading to apoptosis, autophagy and necrosis of tumor cells.4 With the addition of nanoparticles, PS is delivered across the blood-brain barrier, and especially transport drugs to cell chambers such as nuclei.5 This review focuses on the pathological mechanism of PTT and PDT killing tumor tissue.

Diagram 1.1 shows the Benign Tumor & Malignant Tumor
Two-dimensional transition metal dichalcogenide nanomaterials for combination cancer therapy
As demonstrated by preclinical and clinical studies, it is often difficult to eradicate tumors, particularly those that are deep-located, with photothermal therapy (PTT) alone because of the intrinsic drawbacks of optical therapy. To increase the therapeutic effect of PTT and reduce its significant side-effects, a new direction involving the combination of PTT with other therapeutic techniques is highly desirable. Recently, two-dimensional (2D) transition metal dichalcogenides (TMDCs), the typical ultrathin 2D layer nanomaterials, have gained tremendous interest in many different fields including biomedicine, due to their novel physicochemical properties. Benefitting from their intrinsic near-infrared absorbance properties and extremely large specific surface areas, many efforts are being devoted to fabricating 2D TMDC-based multifunctional nanoplatforms for combining PTT with other therapeutics in order to realize 2D TMDC-assisted combination therapy and thus achieve excellent anti-tumor therapeutic efficacy. In addition, various inorganic nanoparticles and fluorescent probes can be attached to the surface of 2D TMDCs to obtain nanocomposites with versatile optical and/or magnetic properties that are useful for multi-modal imaging and imaging-guided cancer therapy. In this review, we mainly summarize the latest advances in the utilization of 2D TMDCs for PTT combination cancer therapy, including PTT/photodynamic therapy, PTT/chemotherapy, PTT/radiotherapy, PTT/gene therapy, and imaging-guided cancer combination therapy, as well as the evaluation of their behaviors and toxicology both in vitro and in vivo. Furthermore, we address the principle for the design of 2D TMDC-assisted photothermal combination theranostics and the future prospects and challenges of using 2D TMDC-based nanomaterials for theranostic applications.6
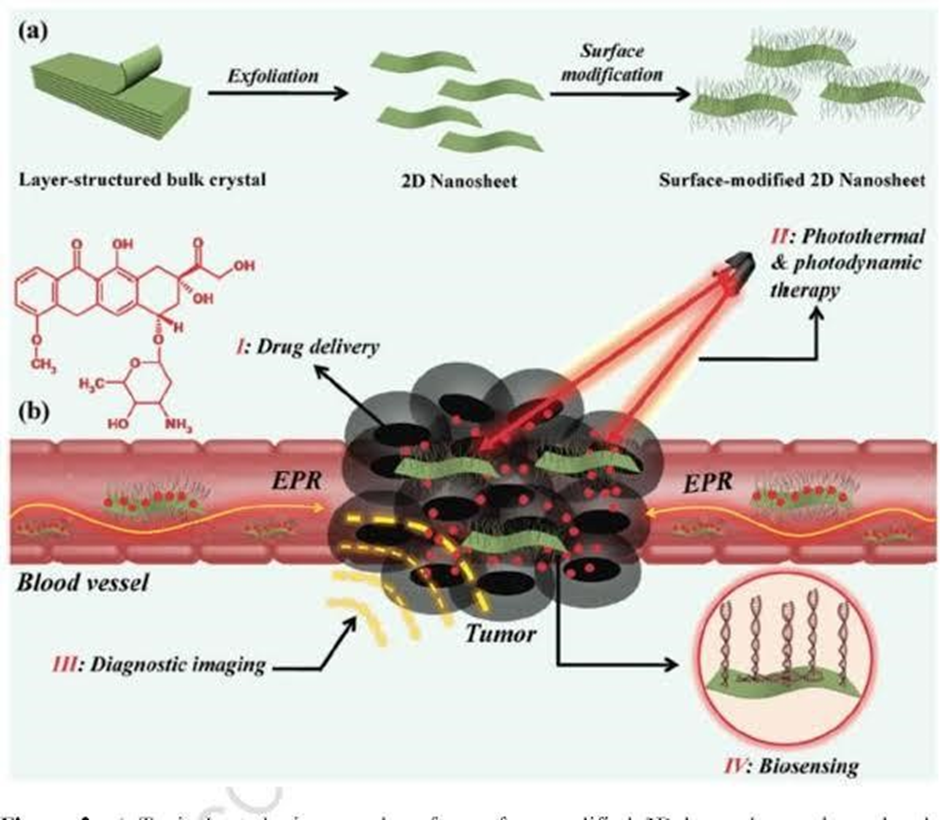
Diagram 2.1 shows the functionalization of 2D transition metal dichalcogenides for biomedical application.
Mechanisms of Photodynamic and Photothermal Therapies
When light interacts with a molecule, one of the phenomena that can occur is absorption. When the molecule absorbs light, it leaves the lowest-energy state, which is called the ground state (PS0), and goes into an excited state with a high energy content (Sn). Within this electronically excited state, there are several sublevels composing the rotational or vibrational states and, by internal conversion phenomenon, coupled with vibrational relaxation, the molecule goes to the lowest excited state (which is called PS1)7.
From the molecule in the S1 state, three different types of energy dissipation can happen: radiative dissipation, vibration relaxation and intersystem crossing (ISC) 8. Among these possibilities to return to S0, molecules release the absorbed energy in the form of light (radiative process). This process is generically called luminescence, and it can be classified as fluorescence or phosphorescence, depending on spin and energy level settings with consequences on the time of transfer8. Fluorescence is when the molecule returns from S1 to S0 directly by radiative emission and is an optical tool widely used in several types of diagnosis and material characterizations9,10.

Diagram 3.1 shows the multifunctional photothermal therapy
The vibrational relaxation is done by intramolecular movements and collisions with the surrounding molecules, resulting in an increase in kinetic energy and, therefore, in the generation of heat. Thermal effects result from the light energy conversion in heat for a combination of non-radiative processes, such as internal conversion, intersystem crossing and vibrational relaxations, necessarily producing mechanical energy11. Then, when thermal energy is generated locally from light irradiation, it is possible to promote thermal damage that ends by leading the cell to death with high selectivity11. PTT uses this route and needs a PA to convert light into heat. The development of efficient PA is essential to guaranteeing the effectiveness of treatments and some important PA properties are infrared absorption for great tissue penetration, good heat conversion efficiency, biocompatibility and non-toxicity in the dark11.
Hyperthermia can also cause indirect cytotoxic effects. Specifically in tumors, the increase in temperature can reduce blood flow and, therefore, lead to hypoxia of tumor cells. These processes make PTT one great new therapeutic option for tumor treatment, when possible, to be done in local matter. Cellular environment temperatures between 42 to 47 °C is enough to make a large number of cells unfeasible, since high temperatures cause the denaturation of proteins, among other effects12. As a selected example, Zhang and co-authors evaluated a gold nanorod as PA against melanoma cells and observed that at 43 °C, the percentages of apoptosis, necroptosis and necrosis of tumor cells were 10.2%, 18.3%, and 17.6%, respectively. Whereas, when the temperature increased 49 °C, necrosis was shown as the dominant cell death pathway (52.8%). Interestingly, when the PTT achieved a moderate temperature of 46?°C, necroptosis was significantly increased (35.1%)13. Moreover, for a PTT protocol, the temperature should be modulated to avoid damage on nearby healthy tissues and intense inflammation response14

Diagram 3.2 shows the mechanism of photodynamic therapy (PDT)
Another technique that has been increasingly accepted in the treatment of various diseases, especially in the treatment of tumors, is PDT. Its mechanism of action involves the interaction of three elements: light at a specific wavelength, a PS, and the presence of molecular oxygen. This interaction results in photo-physical-chemical reactions that generate reactive oxygen species (ROS) with a high oxidizing power of cellular components, making the cell unviable7. For PDT to take place, the molecule goes to its triplet excited state by the so-called intersystem crossing. This event has a low probability according to the selection rules of quantum mechanics and this is a metastable state. From this state it can also return to its ground state by relaxation with the final result in heat or emitting light, which is called phosphorescence. From this metastable state, two types of PDT mechanisms have been proposed to lead to cell death11. The type I mechanism describes the generation of oxidative species, namely hydroxyl radical (•OH), hydrogen peroxide (H2O2), and superoxide ion (O2•?) via electron transfer11.
Differently, in the type II mechanism, the excited PS in the metastable triplet state generated by the ISC undergoes quenching (suppression) by transferring energy directly to the triplet oxygen (3O2), which is in the ground state. Molecular oxygen (in S0), with the energy received, goes to a singlet excited state (1O2), highly cytotoxic and the main mediator of cell damage caused by PDT. In any case, cell death caused by PDT has as its main mechanism of action the production of 1O2 and the induction of cell death by apoptosis. These two mechanisms can happen simultaneously and the proportion between them is directly related to the oxygen concentration in the cellular environment, the intracellular substrates present, and the characteristics of the PSs chosen in the application.
Photothermal Agents (PTAs)
Photothermal agents can convert energy from light into heat to increase the temperature of the surrounding environment. Ideally, Photothermal agents are expected to only increase the temperature locally to reduce the damage to healthy tissues, where the PTA is absent or outside the scope of laser irradiation. Inorganic PTAs own higher PCE and better Photothermal stability than their organic agents. But organic PTAs may win out regarding biodegradability and biocompatibility15

Diagram 4.1 shows the different types of photothermal agents used for PTT.
Transition metal dichalcogenides (TMDCs) are increasingly being used for chemical sensing, biosensing, and tumor therapy on account of their diversity, biocompatibility, multifunctionality, adjustable bandgap, and excellent photoelectric characteristics. This review mainly discusses the effect of the elemental composition and structure of TMDCs on the performance of electrochemical, biofluorescence, and colorimetric biosensors. The applications of TMDCs in tumor therapies are reviewed here. Furthermore, the current challenges and future directions for developing TMDC-based theranostics are summarized16.
Photothermal Transduction Agents
PTAs facilitate light absorption and light conversion to heat. The ideal photothermal conversion agent should have good photostability, an NIR absorption capacity, a high PCE, and good biocompatibility18,19.
PTAs include inorganic materials and organic materials. Inorganic materials include noble metals (e.g., gold nanoparticles and platinum nanoparticles)20,21 carbon-based nanomaterials (e.g., carbon nanotubes, graphene, graphene oxide, carbon quantum dots, and mesoporous carbon)17,20,23,24. Organic materials include NIR-responsive small molecules and semiconducting polymer nanoparticles25,26,27 (e.g., cyanine, porphyrin, copper phthalocyanine, and diketone pyrrole pyrrole).
Generally speaking, inorganic PTAs have higher PCE and better photothermal stability than organic PTAs, but organic PTAs have greater biodegradability and biocompatibility compared to inorganic PTAs28.With a large number of studies on spherical and rod-like morphologies, the exploration of two-dimensional (2D) nanomaterials in the fields of sensing29,30, catalysis31, device manufacturing32, and energy storage33,34 has increased greatly in recent years35.

Diagram 5.1 shows the photodynamic therapy
Common 2D materials include black phosphorus, nanosheets, boron nitride, and graphitic carbon nitride etc. Scientists are trying to overcome the shortcomings of different types of materials so as to further improve the effect of tumor treatment.
Photodynamic Therapy
PDT is also an effective treatment modality for all liver cancers and improves patients’ quality of life.36 Liver cancer mainly includes hepatocellular carcinoma (HCC), cholangiocarcinoma and mixed cancer, and it is the second leading cause of cancer death in the world, second only to lung cancer, with a mortality rate of over 95%, especially in the case of HCC.37 Multiple mechanisms are involved in PDT-mediated tumour cell killing of HCC in vitro and in vivo. Shi et al used sinus porphyrin as a PS in the treatment of HCC, including bel-7402 and HepG2 cell lines, and the results suggested that PDT may be caused by mitochondrial damage, which in turn triggered an apoptotic response, such as cytochrome c release into the cytoplasm, leading to caspase protein activation, etc.38
Antitumor Treatment of PDT and PTT Combined with Immunotherapy
Combination therapy of PDT and PTT is ideal because PDT can increase the sensitivity of tumour cells to PTT by interfering with the TME, disrupting tumour physiology.39 Moreover, the heat generated by PTT can increase blood flow, thereby improving oxygen supply to enhance the therapeutic effect of PDT.40 In addition, the tumour cell debris generated by phototherapy (including PDT and PTT) can act as TAAs and elicit antitumor immune responses to eliminate residual and metastatic cancer cells.39,42 However, the effect of phototherapy is seriously affected by the tissue penetration depth of NIR light, and its antitumour immune effect is inadequate to alleviate the immunosuppression of TME.43 Therefore, the combination of photoimmunotherapy and TME regulation may be one of the ideal strategies to improve the antitumor effect. Zhang et al reported that the combination of hyaluronic acid (HA)-BP, which is a nanoparticle modified by PEG HA, PDT and PTT not only significantly inhibited the original tumour but also induced immunogenic cell death and release DAMPs, thereby inducing the maturation of dendritic cells, activating effector cells and strongly arousing anti-tumour immune responses for cancer therapy.44 Hu et al showed that the synergistic treatment of PTT, PDT and chemo dynamic therapy not only inhibited the growth of primary tumours but also continuously stimulated the anti-tumour immune response of the system, activated T cells and then effectively inhibit tumour metastasis and growth of distant tumours by interacting with PD-1 or PD-L1 checkpoint blockade.

Diagram 6.1 show the combined effect of antitumor
In general, PTT and PDT can enhance the immunotherapy response through the following mechanisms:45 (1) immunogenic cell death induced by PTT and PDT can effectively damage tumours through the action of local immune cells; (2) tumour-specific antigens released by the death of immunogenic cells can be used as in-situ vaccines;46 (3) DAMPs enhance the typical weak immunogenicity of natural tumour antigens;47 (4) proinflammatory cytokines up-regulate and promote the activation of immune system. These effects cooperate with immunotherapy to increase the tumour infiltration of cytotoxic CD8+ and effector memory T cells to effectively eliminate the target tumour and residual cancer cells and trigger immune memory to prevent tumour recurrence and provide the possibility of cure.45,48
PDT
PDT is a clinical approach to treating non-invasive tumours using PSs, oxygen, light and selective photodynamic, and it can be combined with PSs to produce a large amount of reactive oxygen species (ROS) under specific light irradiation wavelength, kill tumour cells and inhibit their growth.49 PDT is widely used in the treatment of solid tumours (such as bladder, oesophageal, skin and breast cancers), vascular, dental and other diseases due to its low toxicity, lack of drug resistance and mild (or no) adverse reactions.50
CONCLUSION
PDT and PTT have a number of advantages, such as lack of drug resistance, minimal trauma, etc., and they have become several of the effective methods to treat cancer and improve the efficacy of clinical treatment. Especially, PSs or photothermal conversion agents synthesised based on nanotechnology can greatly improve the survival rate of patients by combining phototherapy with immunotherapy. However, the therapeutic effect of phototherapy on tumours largely depends on sufficient laser irradiation dose, and the circulation of nanoparticles in the human body is unclear. Moreover, long-term experimental observation of large samples is lacking. Therefore, the potential long-term effects of phototherapy need to be further and continuously studied. At present, most of the schemes are still under exploration, and a certain gap exists in the actual realisation of extensive clinical treatment. With the progress and development of medical treatment, the multi-mode combined treatment scheme will become a new opportunity and challenge for the new generation of cancer treatment.
REFERENCE
- Zhang LX, Sun XM, Xu ZP, Liu RT. Development of multifunctional clay-based nanomedicine for elimination of primary invasive breast cancer and prevention of its lung metastasis and distant inoculation. ACS Appl Mater Interfaces. 2019;11(39):35566–35576. doi: 10.1021/acsami.9b11746 [PubMed] [CrossRef] [Google Scholar]
- Raeesi V, Chou LY, Chan WC. Tuning the drug loading and release of DNAassembled gold-nanorod superstructures. Adv Mater. 2016;28(38):8511–8518. doi: 10.1002/adma.201600773 [PubMed] [CrossRef] [Google Scholar]
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804–827. doi: 10.1097/DSS.0000000000000800 [PubMed] [CrossRef] [Google Scholar]
- Garg AD, Dudek AM, Ferreira GB, et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013;9(9):1292–1307. doi: 10.4161/auto.25399 [PubMed] [CrossRef] [Google Scholar]
- Li Y, Wen T, Zhao R, et al. Localized electric field of plasmonic nanoplatform enhanced photodynamic tumor therapy. ACS Nano. 2014;8(11):11529–11542. doi: 10.1021/nn5047647 [PubMed] [CrossRef] [Google Scholar]
- L Gong, Two-dimensional transition metal dichalcogenide in the utilization of 2D TMDCs for PTT combination cancer therapy; 2017 by [RSC Publishing].
- Wilson, B.C.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008, 53, R61–R109. [Google Scholar] [CrossRef]
- Feng, G.X.; Zhang, G.Q.; Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhao, Y.L. Applications of Light-Responsive Systems for Cancer Theranostics. Acs Appl. Mater. Interfaces 2018, 10, 21021–21034. [Google Scholar] [CrossRef]
- Rozanova, N.; Zhang, J.Z. Photothermal ablation therapy for cancer based on metal nanostructures. Sci. China Ser. B-Chem. 2009, 52, 1559–1575. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhan, X.L.; Xiong, J.; Peng, S.S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.L.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef][Green Version]
- Gao, P.; Wang, H.; Cheng, Y. Strategies for efficient photothermal therapy at mild temperatures: Progresses and challenges. Chin. Chem. Lett. 2021, 7, 48–59. [Google Scholar]
- Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chemical Society Reviews. 2019; 48(7):2053-108.
- Lude Wang, Duo Xu, Lianfu Jiang, Jie Gao, Zhongmin Tang, Yunjie Xu, Xiang Chen, Han Zhang First published: 08 October 2020 https://doi.org/10.1002/adfm.202004408.
- Zhang, P.; Hu, C.; Ran, W.; Meng, J.; Yin, Q.; Li, Y. Recent Progress in Light-Triggered Nanotheranostics for Cancer Treatment. Theranostics 2016, 6, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Mao, L.; Dong, Y.; Zhao, Z.; Sun, Q.; Mazhar, M.; Ma, Y.; Yang, S.; Ren, W. Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy. Pharmaceutics2021, 13, 2070. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Li, J.; Chen, Y.; Chen, Y.; Kawazoe, N.; Chen, G. Bifunctional scaffolds for the photothermal therapy of breast tumor cells and adipose tissue regeneration. J. Mater. Chem. B 2018, 6, 7728–7736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, Y.; Yang, T.; Wang, Q.; Lv, X.; Song, X.; Ke, H.; Guo, Z.; Huang, X.; Hu, J.; Li, Z.; et al. Albumin-coordinated assembly of clearable platinum nanodots for photoinduced cancer theranostics. Biomaterials 2018, 154, 248–260. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, X.; Shi, J.; Jiang, Z.; Zhang, C.Y. Graphene-based nanomaterials for cancer therapy and anti-infections. Bioact. Mater. 2022, 14, 335–349. [Google Scholar] [CrossRef]
- Lagos, K.J.; Buzzá, H.H.; Bagnato, V.S.; Romero, M.P. Carbon-Based Materials in Photodynamic and Photothermal Therapies Applied to Tumor Destruction. Int. J. Mol. Sci. 2021, 23, 22. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J.; Jiang, K.; Tang, Z.; Wang, Y.; Li, Z.; Liu, C.; Wu, A.; Lin, H. Ce6Modified Carbon Dots for Multimodal-Imaging-Guided and Single-NIR-Laser-Triggered Photothermal/Photodynamic Synergistic Cancer Therapy by Reduced Irradiation Power. ACS Appl. Mater. Interfaces 2019, 11, 5791–5803. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109891. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Chang, Y.; Sun, C.; Shao, S.; Xie, Z.; Xiao, X.; Ma, P.; Zhang, H.; Cheng, Z.; et al. Intelligent Hollow Pt-CuS Janus Architecture for Synergistic CatalysisEnhanced Sonodynamic and Photothermal Cancer Therapy. Nano Lett. 2019, 19, 4134– 4145. [Google Scholar] [CrossRef]
- Curcio, A.; Silva, A.K.A.; Cabana, S.; Espinosa, A.; Baptiste, B.; Menguy, N.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- Ling, C.; Wang, X.; Shen, Y. Advances in Hollow Inorganic Nanomedicines for Photothermal-Based Therapies. Int. J. Nanomed. 2021, 16, 493–513. [Google Scholar] [CrossRef]
- Chi, J.; Li, J.; Ren, S.; Su, S.; Wang, L. Construction and Application of DNA-twodimensional Layered Nanomaterials Sensing Platform. Acta Chim. Sin. Chin. Ed. 2019, 77, 1230–1238. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Ge, Y.; Shao, Y. Two-dimensional nanomaterials for Frster resonance energy transfer–based sensing applications. Nanophotonics2020, 9, 1855–1875. [Google Scholar] [CrossRef]
- Lan, G.; Quan, Y.; Wang, M.; Nash, G.T.; You, E.; Song, Y.; Veroneau, S.S.; Jiang, X.; Lin, W. Metal-Organic Layers as Multifunctional Two-Dimensional Nanomaterials for Enhanced Photoredox Catalysis. J. Am. Chem. Soc.2019, 141, 15767–15772. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, L.; Liu, K.; Liu, S.; Li, X.; Zhang, Q.; Shuai, J. Two-Dimensional Nanomaterials for Boosting the Performance of Organic Solar Cells. Coatings 2021, 11, 1530. [Google Scholar] [CrossRef]
- Liu, J.; Hao, R.; Jia, B.; Zhao, H.; Guo, L. Manipulation on Two-Dimensional Amorphous Nanomaterials for Enhanced Electrochemical Energy Storage and Conversion. Nanomaterials 2021,11, 3246. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Gao, L.; Li, T.; Mei, S.; Wang, C.; Wen, B.; Huang, W.; Li, C.; Zheng, G.; Wang, H.; et al. Two-Dimensional Black Phosphorus Nanomaterials: Emerging Advances in Electrochemical Energy Storage Science. Nanomicro lett. 2020, 12, 179. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. TwoDimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Correction: Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Zou H, Wang F, Zhou JJ, et al. Application of photodynamic therapy for liver malignancies. J Gastrointest Oncol. 2020;11(2):431–442. doi: 10.21037/jgo.2020.02.10 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [PubMed] [CrossRef] [Google Scholar]
- Shi R, Li C, Jiang Z, et al. Preclinical Study of Antineoplastic Sinoporphyrin SodiumPDT via In Vitro and In Vivo Models. Molecules. 2017;22(1):112. doi: 10.3390/molecules22010112 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Xu J, Xu L, Wang C, et al. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano. 2017;11(5):4463–4474. doi: 10.1021/acsnano.7b00715 [PubMed] [CrossRef] [Google Scholar]
- 163. Qiu J, Xiao Q, Zheng X, et al. Single W18O49 nanowires: a multifunctional nanoplatform for computed tomography imaging and photothermal/photodynamic/radiation synergistic cancer therapy. Nano Res. 2015;8(11):3580–3590. doi: 10.1007/s12274-015-0858-z [CrossRef] [Google Scholar]
- Wang H, Pan X, Wang X, et al. Degradable Carbon-Silica Nanocomposite with Immunoadjuvant Property for Dual-Modality Photothermal/Photodynamic Therapy. ACS Nano. 2020;14(3):2847–2859. doi: 10.1021/acsnano.9b06168 [PubMed] [CrossRef] [Google Scholar]
- Liang R, Liu L, He H, et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149–160. doi: 10.1016/j.biomaterials.2018.05.051 [PubMed] [CrossRef] [Google Scholar]
- Hameed S, Mo S, Mustafa G, et al. Immunological Consequences of NanoparticleMediated Antitumor Photoimmunotherapy. Adv Therapeutics. 2020;3(5):1900101. doi: 10.1002/adtp.201900101 [CrossRef] [Google Scholar]
- Zhang X, Tang J, Li C, et al. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact Mater. 2021;6(2):472–489. doi: 10.1016/j.bioactmat.2020.08.024 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2 [PubMed] [CrossRef] [Google Scholar]
- 169. Naylor MF, Chen WR, Teague TK, Perry LA, Nordquist RE. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br J Dermatol. 2006;155(6):1287–1292. doi: 10.1111/j.1365-2133.2006.07514.x [PubMed] [CrossRef] [Google Scholar]
- Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol. 2011;7(1):75–91. doi: 10.1586/eci.10.81 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Santos LL, Oliveira J, Monteiro E, Santos J, Sarmento C. Treatment of head and neck cancer with photodynamic therapy with redaporfin: a clinical case report. Case Rep Oncol. 2018;11(3):769–776. doi: 10.1159/000493423 [PMC free article][PubMed] [CrossRef] [Google Scholar]
- Gong H, Chao Y, Xiang J, et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016;16(4):2512–2521. doi: 10.1021/acs.nanolett.6b00068 [PubMed] [CrossRef] [Google Scholar]
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145–163. doi: 10.2147/CCID.S35334 [PMC free article] [PubMed] [CrossRef] [Google Scholar]


 Wasif Rao*
Wasif Rao*
 Bishal Singh
Bishal Singh
 Asif
Asif
 Robin Kumar
Robin Kumar







 10.5281/zenodo.12798987
10.5281/zenodo.12798987